Attorney Docket No. TIZI-035/001WO 322161-2568 COMBINATIONS OF FORALUMAB WITH GLUCAGON-LIKE PEPTIDE 1 (GLP-1) AGONISTS OR SODIUM-GLUCOSE COTRANSPORTER-2 (SGLT2) INHIBITORS AND METHODS OF USE THEREOF CROSS-REFERENCE TO RELATED APPLICATIONS [0001] The present application claims the benefit of U.S. Provisional Patent Application Nos.63/610,355, filed December 14, 2023, and No.63/712,058, filed October 25, 2024, each of which is incorporated herein by reference in its entirety. INCORPORATION-BY-REFERENCE OF SEQUENCE LISTING [0002] The Sequence Listing XML associated with this application is provided electronically in XML file format and is hereby incorporated by reference into the specification. The name of the XML file containing the Sequence Listing XML is TIZI- 035_001WO_SeqList.xml”. The XML file is 10,659 bytes, created on December 2, 2024, and is being submitted electronically via USPTO Patent Center. F
IELD [0003] This disclosure relates to composition and methods for the treatment, prevention and reduction of a comorbidity associated with chronic inflammation such as obesity, Type 1 Diabetes (T1), Type 2 Diabetes (T2D), metabolic syndrome, chronic kidney disease, and cardiovascular risk, as well as methods for improving the efficacy of GLP-1 agonists. BACKGROUND [0004] Short-term inflammation is an important body process that helps us fight off infection and injuries, the chronic inflammation is known to be harmful to our health and can set the stage for obesity, metabolic syndrome, diabetes and cardiovascular risk. See, e.g., Donath et al., Nature Reviews (2019) 19:734-746; Candia et al. Frontiers in Immunology (2019) Vol 10 Article 451. Diabetes is the world's eighth biggest killer, accounting for some 1.5 million deaths each year. A major new World Health Organization report has now revealed that the number of cases around the world has nearly quadrupled to 422 million in 2014 from 108 million in 1980. The Eastern-Mediterranean region had the biggest increase in cases during that time frame. Diabetes now affects one in 11 adults with high blood sugar levels linked to 3.8 million deaths every year.
Attorney Docket No. TIZI-035/001WO 322161-2568 [0005] Obesity, a pervasive global epidemic, is closely associated with several chronic metabolic disorders, such as type 2 diabetes (T2D) and metabolic-associated fatty liver disease (MAFLD), which can progress into metabolic-associated steatohepatitis (MASH) (Tilg et al. Nat Rev Gastroentero 14, 32–42 (2017). Although current treatments like glucagon-like peptide-1 receptor agonists (GLP-1RAs) are effective in reducing weight and improving glycemic control, their long-term utility is limited by adverse effects and diminished efficacy in the later stages of these conditions (Müller et al. Nat. Rev. Drug Discov.21, 201–223 (2022). This limitation highlights a critical need for new therapeutic approaches that not only focus on glucose regulation and weight management but also directly address the ongoing inflammation and tissue damage seen in patients with chronic metabolic diseases. [0006] Chronic inflammation and fibrosis in metabolic tissues, especially the liver and adipose tissue, manifest despite controlled systemic glucose levels, suggesting that metabolic homeostasis alone is insufficient to reverse long-term tissue degeneration observed in MAFLD and MASH (Soták et al. Nat. Rev. Endocrinol.1–17 (2024) doi:10.1038/s41574- 024-01047-y). This observation points to the critical role of the immune system, particularly T cells, in mediating these complications. In both humans and mice, T cells are pivotal in modulating inflammation and insulin resistance in adipose tissue and drive fibrosis in the liver, where the expansion and activation of pro-inflammatory Th1, Th17, and CD8 T cells promote disease progression, whereas the increase of Tregs and Th2 and their production of IL-10, IL4, and IL-13 promoted anti-inflammatory macrophages, essential for restoring tissue repair and homeostasis (Valentine et al. Immunol. Rev. (2024) doi:10.1111/imr.13354; Sutti et al. Nat Rev Gastroentero 17, 81–92 (2020)). [0007] GLP-1 agonists and SGLT-2 inhibitors have been shown to help control blood sugar and boost weight loss. GLP-1s and SGLT-2 inhibitors also have other major benefits. Research has found that some drugs in these groups may lower the risk of heart disease, such as heart failure, stroke, major adverse cardiac events (MACE) and kidney disease. People taking these drugs have seen their blood pressure and cholesterol levels improve. [0008] Antibodies to the CD3 epsilon signaling molecule of the T-cell receptor complex, such as Foralumab have proven to be effective as an immunoregulatory agent and offer the potential co-therapy in the treatment, prevention and the reduction of comorbidity associated with chronic inflammation. Obesity is linked to various neurological disorders that targeted by anti-CD3, and addressing obesity may not only improve obesity-related complications, like type 2 diabetes, but also other related disorders. Anti-CD3 antibodies
Attorney Docket No. TIZI-035/001WO 322161-2568 have been shown to improve obesity-induced insulin resistance. Winer et al., Nature Medicine (2009) 15(8):921-929. However, anti-CD3 antibodies were not able to improve glucose metabolism in mouse models. Ilan et al., Proc Nat Acad Sci. (2010) 107(21):9765- 70. [0009] Available agents to treat diabetes have several limitations, including that the treatment is symptomatic and not therapeutic and they are not effective to stop tissue damage directly, since their effects on inflammation are indirect. Moreover, some available agents are shown to be immunosuppressive. Previous therapeutic interventions, such as intraperitoneal (i.p.) administration of anti-CD3 antibodies (anti-CD3 antibody), have targeted T cell modulation but were frequently associated with severe systemic side effects, limiting their widespread adoption (Soták et al. Nat. Rev. Endocrinol.1–17 (2024) doi:10.1038/s41574-024-01047-y). Additionally, while oral administration of anti-CD3 antibody has been successful in inducing regulatory T cells (Tregs) and promoting immune tolerance in many inflammatory and auto-immune preclinical contexts, it was shown that oral tolerance mechanisms are often compromised in conditions of diet-induced obesity. This approach leverages the unique properties of the mucosal immune system to modulate T cell activity selectively, aiming to minimize the limitations seen with systemic and oral administrations. [00010] Thus, there remains an unmet need for novel therapies to treat the pathogenesis of diabetes, as well as to improve the efficacy and safety of GLP-1 agonists such as semaglutide. SUMMARY [00011] In one aspect, provided herein is a method of treating an obesity-related complication in a subject in need thereof, comprising administering to the subject a GLP-1 agonist and foralumab. In some embodiments, the obesity-related complication is inflammation. In some embodiments, the inflammation is liver inflammation, adipose tissue inflammation, or systemic inflammation. In some embodiments, the obesity-related complication is liver damage. In some embodiments, the obesity-related complication is fibrosis. In some embodiments, the obesity-related complication is a systemic metabolism abnormality. In some embodiments, the systemic metabolism abnormality is an increase in the blood levels of total cholesterol, lactate dehydrogenase (LDH), triglycerides, aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN),
Attorney Docket No. TIZI-035/001WO 322161-2568 amylase, and/or lipases. In some embodiments, the obesity-related complication is sarcopenia. [00012] In some embodiments, the GLP-1 agonist is a semaglutide. In some embodiments, the semaglutide is Ozempic®, Wegovy® or Rybelsus®. [00013] In some embodiments, the foralumab is administered prior to the GLP-1 agonist. In some embodiments, the foralumab and the GLP-1 agonist are administered simultaneously. In some embodiments, the foralumab is administered concurrently with the GLP-1 agonist for a first period of time and subsequently the foralumab is administered in the absence of the GLP-1 agonist for a second period of time. In some embodiments, the GLP-1 agonist is administered subcutaneously. In some embodiments, the foralumab is administered intranasally. [00014] In another aspect, provided herein is a method of improving the efficacy of a GLP-1 agonist in a subject receiving the GLP-1 agonist, comprising administering to the subject foralumab. In some embodiments, the improvement in efficacy is a decrease in inflammation. In some embodiments, the inflammation is liver inflammation, adipose tissue inflammation, or systemic inflammation. In some embodiments, the improvement in efficacy is a decrease in liver damage. In some embodiments, the improvement in efficacy is a decrease in fibrosis. In some embodiments, the improvement in efficacy is an improvement in liver homeostasis. In some embodiments, the improvement in efficacy is an improvement in adipose homeostasis. In some embodiments, the improvement in efficacy is a decrease in lipid accumulation. In some embodiments, the improvement in efficacy is an increase in brown adipose tissue thermogenesis. In some embodiments, the improvement is an improvement in one or more biomarkers of systemic lipid metabolism. In some embodiments, the biomarker of lipid metabolism is AST, ALT, BUN, cholesterol, LDH or triglycerides. In some embodiments, the improvement in efficacy is an increase in liver regeneration. In some embodiments, the improvement is a reduction in sarcopenia. [00015] In some embodiments, the foralumab is administered prior to the GLP-1 agonist. In some embodiments, the foralumab and the GLP-1 agonist are administered simultaneously. In some embodiments, the foralumab is administered concurrently with the GLP-1 agonist for a first period of time and subsequently the foralumab is administered in the absence of the GLP-1 agonist for a second period of time. In some embodiments, the GLP-1 agonist is administered subcutaneously. In some embodiments, the foralumab is administered intranasally.
Attorney Docket No. TIZI-035/001WO 322161-2568 [00016] In another aspect, provided herein is a method for the treatment of chronic inflammation in a subject, the method comprising administering to the subject (1) foralumab and (2) a GLP-1 agonist or an SGLT-2 inhibitor. In some embodiments, the chronic inflammation is secondary to obesity, Type 1 Diabetes (T1), Type 2 Diabetes (T2D), metabolic syndrome, chronic kidney disease or cardiovascular disease. In some embodiments, the foralumab is administered nasally. In some embodiments, the subject is administered foralumab and a GLP-1 agonist, and wherein the GLP-1 agonist is administered by injection. In some embodiments, the subject is administered foralumab and a GLP-1 agonist, and wherein the GLP-1 agonist is Dulaglutide, Exenatide extended release, Exenatide Semaglutide, Liraglutide, and Lixisenatide. In some embodiments, the subject is administered foralumab and an SGLT-2 inhibitor, and wherein the SGLT-2 inhibitor is administered orally. In some embodiments, the subject is administered foralumab and an SGLT-2 inhibitor, and wherein the SGLT-2 inhibitor is Brenzavvy® (bexaglifloxin),. Invokana® (canagliflozin), Farxiga® (dapagliflozin), Jardiance® (empagliflozin), or Steglatro® (ertugliflozin). In some embodiments, the subject has a body mass index (BMI) of greater than or equal to 25. In some embodiments, the subject has heart failure with preserved injection fraction. BRIEF DESCRIPTION OF THE DRAWINGS [00017] FIG.1 illustrates the experimental design to assess the impact of nasal anti- CD3 on whole-body metabolism in diet induced obese mice. C57BL/6 males def with HFD for 12 weeks (start at age 6 weeks) were treated with 1 ug nasal anti-CD3 antibody or with isotype control three times a week for 6 weeks. [00018] FIGs.2A and 2B are representative graphs showing body weight and food consumption, respectively in mice treated with anti-CD3 antibody or isotype control. [00019] FIGs.3A-3C are dot plots illustrating that nasal anti-CD3 ameliorates locomotor activity. [00020] FIG.4 is a graph of CLAMS measurement f energy expenditure during the last 48 hours of day and light cycles of 6 weeks treatment. [00021] FIG.5 shows the respiratory exchange rate (RER) during the last 48 hours of day and light cycles of 6 weeks treatment. [00022] FIGs.6A-6C are representative graphs of glucose levels during glucose tolerance test (GTT), with area under the curve (AUC) (FIG.6A), insulin tolerance test (FIG. 6B) and fasting insulin levels (FIG.6C). *p <0.05.
Attorney Docket No. TIZI-035/001WO 322161-2568 [00023] FIG.7 shows that nasal anti-CD3 ameliorates lipid handling in DIO mice. *p <0.05, **p <0.01. [00024] FIG.8 shows that nasal anti-CD3 ameliorates liver, kidney and pancreatic function. *p <0.05, **p <0.01. [00025] FIG.9 shows the effect of nasally administered anti-CD3 antibody (aCD3; lower panel) compared to controls (PBS; top) on liver damage in DIO mice. [00026] FIGs.10A-10D show how nasal anti-CD3 antibody (aCD3) restores adipose tissue homeostasis in a diet-induced obesity model. FIG.10A shows magnetic resonance imaging (MRI) to measure body fat expansion (subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT). FIG.10B shows histopathological analysis of H&E staining of VAT, with measurements of adipocytes size, and crown like structures (top and bottom right). FIG.10C shows KEGG pathway analysis of RNAseq on total VAT. FIG.10D shows relative gene expression measured by qRT-PCR on total VAT. Mean±SEM. *p <0.05, **p <0.01, ***p <0.001. [00027] FIGs.11A-11D show how nasal anti-CD3 antibody (aCD3) reestablishes hepatic homeostasis in diet-induced obesity. FIG.11A shows spectroscopy measurement of fat to water ratio in the mouse liver in vivo. FIG.11B shows H&E analysis of the accumulation of lipids in the mouse liver. FIGs.11C and 11D show upregulated and downregulated KEGG pathways in total liver tissue analyzed by RNAseq, respectively. Mean±SEM. *p <0.05, **p <0.01, ***p <0.001. [00028] FIG.12 is a heatmap of differential gene expression analyzed by RNAseq on livers collected from lean healthy chow-fed controls age-matched with DIO mice treated with anti-CD3 antibody or PBS vehicle for the indicated periods. [00029] FIG.13 shows the effect of body weight tracking throughout treatment in DIO mice treated with anti-CD3 antibody (aCD3) only or with anti-CD3 antibody in combination with high dose (HD) or low dose (LD) semaglutide (Sema). Nasal Anti-CD3 did not affect semaglutide-induced weight loss in this mouse model. [00030] FIG.14 shows MRI measurements of T1, uptake of oxidative stress probe (Fe³⁺-PyC3A) calculated by ∆CNR, and fatty fractions (FF). [00031] FIG.15 shows representative livers photographs and H&E staining for liver histopathological evaluation of the livers of mice treated with anti-CD3 (aCD3) antibody and/or semaglutide (Sema) in high doses (HD) or low doses (LD), as well as histopathological scoring of hematoxylin and eosin-stained formalin fixed paraffin embedded
Attorney Docket No. TIZI-035/001WO 322161-2568 liver sections. * p ≤ 0.05; ** p ≤ 0.01; *** 0.001. HFD: High-date diet; SubC: subcutaneous; Chow: chow-fed mouse. [00032] FIG.16 shows O red oil staining and quantification of lipid content in hepatocytes in the livers of mice treated with anti-CD3 antibody (aCD3) and/or semaglutide (Sema) in high doses (HD) or low doses (LD) compared to HFD PBS and NaCl control group. * p ≤ 0.05; ** p ≤ 0.01; *** 0.001. HFD: High-date diet; SubC: subcutaneous; Chow: chow-fed mouse. [00033] FIG.17 is a heatmap showing comparative differential gene expression of livers isolated from DIO mice with indicated treatments. [00034] FIG.18 shows immunohistochemical staining with F4/80 of macrophages to highlight crown-like structures and their quantification within VAT of DIO mice with the indicated treatments. HFD: High-fat diet; SubC: subcutaneous; Chow: chow-fed mouse aCD3: anti-CD3 antibody; Sema: semaglutide; HD: high dose; LD: low dose. [00035] FIG.19 is a heatmap showing comparative differential gene expression of VAT isolated from DIO mice with indicated treatments. [00036] FIG.20 shows immunohistochemical staining with Ucp-1 and quantification within BAT of DIO mice with the indicated treatments. HFD: High-fat diet; SubC: subcutaneous; Chow: chow-fed mouse; aCD3: anti-CD3 antibody; Sema: semaglutide; HD: high dose; LD: low dose. [00037] FIGs.21A-21C show the serum levels of TNFα, IL-1β and Keratinocyte chemoattractant (KC)/human growth-regulated oncogene (GRO) (KC/GRO), respectively, in serum samples from mice treated with treated with high dose (HD) or low dose (LD) semaglutide (Sema) alone and/or anti-CD3 antibody (aCD3). * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; **** p ≤ 0.0001. HFD: High- fat diet; SubC: subcutaneous; Chow: chow-fed mouse. [00038] FIGs.22A-22E show expression levels of AST, BUN, cholesterol, LDH, and triglycerides, respectively, in serum samples from mice treated with treated with high dose (HD) or low dose (LD) semaglutide (Sema) alone and/or anti-CD3 antibody (aCD3). * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; **** p ≤ 0.0001. HFD: High-fat diet; SubC: subcutaneous; Chow: chow-fed mouse. [00039] FIG.23 shows Sirius Red IHC staining and scoring of livers isolated from MASH mice or healthy lean age-matched controls. Heatmap showing comparative differential gene expression of livers. * p ≤ 0.05; *** p ≤ 0.001. MASH: MASH (metabolic
Attorney Docket No. TIZI-035/001WO 322161-2568 associated steatohepatitis) diet; SubC: subcutaneous; Chow: chow-fed mouse. H&E: Hematoxylin and eosin; aCD3: anti-CD3 antibody; Sema: semaglutide; HD: high dose. [00040] FIG.24 shows smooth muscle actin alpha (SMAα) staining and quantification of livers isolated from MASH mice or healthy lean age-matched controls. Heatmap showing comparative differential gene expression of livers. * p ≤ 0.05; *** p ≤ 0.001. MASH: MASH (metabolic associated steatohepatitis) diet; SubC: subcutaneous; Chow: chow-fed mouse. H&E: Hematoxylin and eosin; ; aCD3: anti-CD3 antibody; Sema: semaglutide; HD: high dose. [00041] FIGs.25A and 25B are heatmaps showing comparative differential gene expression of livers (FIG.25A) and VAT (FIG.25B) isolated from MASH mice or healthy age matched lean controls treated with indicated treatments. [00042] FIG.26 shows levels of CD45+/IL10+ liver cells in mice treated with semaglutide (Sema) and/or anti-CD3 antibody (aCD3). **** p ≤ 0.0001. MASH: metabolic associated steatohepatitis; SubC: subcutaneous; Chow: chow-fed mouse. [00043] FIGs.27A and 27B shows a Venn diagram of upregulated and downregulated gene expression pathways, respectively, analyzed by Ingenuity Pathway Analysis (IPA) of liver function. aCD3: anti-CD3 antibody; Sema: semaglutide. [00044] FIGs.28A-28C show serum levels of lipid metabolism and liver function markers ALT, cholesterol and triglycerides, respectively, in the serum of mice treated with semaglutide (Sema) and/or anti-CD3 antibody (aCD3). ** p ≤ 0.01; *** p ≤ 0.001; MASH: metabolic associated steatohepatitis; SubC: subcutaneous; Chow: chow-fed mouse; HD: high dose. [00045] FIGs.29A-29C show serum levels of inflammatory markers TNFα, IL-1β and KC/GRO, respectively, in the serum of mice treated with semaglutide (Sema) and/or anti- CD3 antibody (aCD3). * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; **** p ≤ 0.0001. MASH: metabolic associated steatohepatitis diet; SubC: subcutaneous; Chow: chow-fed mouse; HD: high dose. [00046] FIGs.30A-30F show nasal anti-CD3-induced T cell modulation reconfigures myeloid compartments to support tissue homeostasis in obesity-related pathologies. Shown are representative flow cytometry plots and quantification of Tregs in spleen and adipose tissue (VAT) (FIG.30A), and of CD8 and Th17 (RORgt+CD127+) in livers isolated from DIO mice treated with nasal anti-CD3 antibody (anti-CD3) or isotype control (Iso) for 6 weeks (FIG.30B). Proportions of pro-inflammatory macrophages (M1-like) to anti-
Attorney Docket No. TIZI-035/001WO 322161-2568 inflammatory and tissue remodeling (M2-like) macrophages in VAT (FIG.30C), neutrophils (FIG.30D) and inflammatory monocyte-derived macrophages (FIG.30E) in livers isolated from DIO mice treated with nasal anti-CD3 antibody or isotype for 6 weeks. FIG.30F shows a representative flow cytometry plots and quantification of Kupffer cells and monocytes- derived macrophages identified as Tim4
+ and Tim4- MHC class II
+CD11b
+F4/80
high respectively; MASH: metabolic associated steatohepatitis diet; SubC: subcutaneous; Sema; semaglutide; HD: high dose. [00047] FIGs.31A and 31B show decreases in inflammatory cells in livers of mice treated with anti-CD3 antibody (aCD3) compared to isotype controls (Iso). FIGs.31C and 31D show levels of ALT and BUN after prophylactic and therapeutics treatment with the nasal anti-CD3 antibody. DETAILED DESCRIPTION [00048] The present disclosure provides compositions and methods for the treatment, prevention and reduction of comorbidities associated with chronic inflammation. Comorbidities associated with chronic inflammation include for example obesity, Type 1 Diabetes (T1D), Type 2 Diabetes (T2D), metabolic syndrome, chronic kidney disease, and cardiovascular risk. [00049] Specifically, the disclosure provides administration of nasal formulations of Foralumab, an anti- CD3ε antibody shown to reduce inflammation together with either a GLP-1 agonist or a sodium-glucose cotransporter-2 (SGLT 2) inhibitor for the treatment, prevention and reduction of comorbidities associated with inflammation. The GLP-1 agonist may be a dual agent, for example, a dual GLP-1 agonist and glucose-dependent insulinotropic polypeptide (GIP) receptor agonist. CD3 Antibodies [00050] The present disclosure provides formulation for nasal delivery of Foralumab, an antibody specific against the CD3 epsilon chain (CD3ε). [00051] Foralumab comprises a heavy chain complementarity determining region 1 (CDRH1) comprising the amino acid sequence GYGMH (SEQ ID NO: 1), a heavy chain complementarity determining region 2 (CDRH2) comprising the amino acid sequence VIWYDGSKKYYVDSVKG (SEQ ID NO: 3), a heavy chain complementarity determining region 3 (CDRH3) comprising the amino acid sequence QMGYWHFDL (SEQ ID NO: 4), a light chain complementarity determining region 1 (CDRL1) comprising the amino acid
Attorney Docket No. TIZI-035/001WO 322161-2568 sequence RASQSVSSYLA (SEQ ID NO: 5), a light chain complementarity determining region 2 (CDRL2) comprising the amino acid sequence DASNRAT (SEQ ID NO: 6), and a light chain complementarity determining region 3 (CDRL3) comprising the amino acid sequence QQRSNWPPLT (SEQ ID NO: 7). [00052] Foralumab comprises a variable heavy chain comprising the amino acid sequence QVQLVESGGGVVQPGRSLRLSCAASGFKFSGYGMHWVRQAPGKGLEWVAVIWYD GSKKYYVDSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCARQMGYWHFDLW GRGTLVTVSS (SEQ ID NO: 8) and a variable light chain comprising the amino acid sequence EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIYDASNRATGI PARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRSNWPPLTFGGGTKVEIK (SEQ ID NO: 9). [00053] Foralumab comprises a heavy chain comprising the amino acid sequence: QVQLVESGGGVVQPGRSLRLSCAASGFKFSGYGMHWVRQAPGKGLEWVAVIWYD GSKKYYVDSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCARQMGYWHFDLW GRGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTS GVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKRVEPKSCDK THTCPPCPAPEAEGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEK TISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNY KTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK (SEQ ID NO: 10) and a light chain comprising the amino acid sequence EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIYDASNRATGI PARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRSNWPPLTFGGGTKVEIKRTVAAPSV FIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDST YSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTKSFNRGEC (SEQ ID NO: 11). [00054] Foralumab may also be referred to herein as NI-0401, or 28F11-AE. (See e.g., Dean Y, Dépis F, Kosco‐Vilbois M. “Combination therapies in the context of anti‐CD3 antibodies for the treatment of autoimmune diseases.” Swiss Med Wkly. (2012) (the contents of which are hereby incorporated by reference in its entirety).
Attorney Docket No. TIZI-035/001WO 322161-2568 Formulations [00055] Foralumab may be formulated in any suitable excipient. In some embodiments, the foralumab formulation can be a liquid. In some embodiments, the liquid formulation is aqueous. [00056] The formulation may include one or more salts (a buffering salt), one or more polyols and one or more excipients. [00057] The formulations may also contain buffering agents, or preservatives. As used in this application, the terms "buffer" or "buffer system" is meant a compound that, usually in combination with at least one other compound, provides a buffering system in solution that exhibits buffering capacity, that is, the capacity to neutralize, within limits, either acids or bases (alkali) with relatively little or no change in the original pH. [00058] Buffers include borate buffers, phosphate buffers, calcium buffers, and combinations and mixtures thereof. Borate buffers include, for example, boric acid and its salts, for example, sodium borate or potassium borate. Borate buffers also include compounds such as potassium tetraborate or potassium metaborate that produce borate acid or its salt in solutions. [00059] A phosphate buffer system includes one or more monobasic phosphates, dibasic phosphates and the like. Particularly useful phosphate buffers are those selected from phosphate salts of alkali and/or alkaline earth metals. Examples of suitable phosphate buffers include one or more of sodium dibasic phosphate (Na2HPO4), sodium monobasic phosphate (NaH2PO4) and potassium monobasic phosphate (KH2PO4). The phosphate buffer components frequently are used in amounts from 0.01% or to 0.5% (w/v), calculated as phosphate ion. [00060] Other known buffer compounds can optionally be added to the according to the formulations, for example, citrates, sodium bicarbonate, TRIS, and the like. Other ingredients in the solution, while having other functions, may also affect the buffer capacity. For example, EDTA, often used as a complexing agent, can have a noticeable effect on the buffer capacity of a solution. [00061] The formulation may be buffered in a solution at a pH in the range of about 4 to 8; in the range of about 4 to 7; in the range of about 4 to 6; in the range of about 5 to 6; or in the range of about 5.5 to 6.5. Preferably, the pH is 5.5. [00062] Any suitable salt may be used in the formulations disclosed herein. Examples of salts that may be present in the formulations disclosed herein include those prepared from the following acids: hydrochloric, hydrobromic, sulfuric, nitric, phosphoric, maleic, acetic,
Attorney Docket No. TIZI-035/001WO 322161-2568 salicylic, citric, boric, formic, malonic, succinic, and the like. Such salts can also be prepared as alkaline metal or alkaline earth salts, such as sodium, potassium or calcium salts. Examples of buffering agents include phosphate, citrate, acetate, and 2-(N- morpholino)ethanesulfonic acid (MES). [00063] Preferred salts for use in the formulation include sodium chloride, sodium acetate, sodium acetate trihydrate and sodium citrate. [00064] In some embodiments, the concentration of salt in the formulations according to the disclosure is between about 10 mM and 500mM, between about 25m and 250 mM, between about 25nM and 150mM. [00065] In some embodiments, the sodium acetate trihydrate is present in the formulation at a concentration in the range of about 10 mM to 100 mM. For example, the sodium acetate trihydrate may be present at a concentration of about 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 95 or 100 mM. Preferably, the sodium acetate trihydrate is present in the formulation at a concentration of 25mM. [00066] In some embodiments, the sodium chloride is present in the formulation at a concentration in the range of about 50 mM to 500 mM. For example, the sodium chloride may be present in the formulation at a concentration of about 50, 55, 60, 65, 70, 75, 80, 85, 90, 95, 100.125, 150, 175, 200, 225, 250, 275, 300, 325, 350, 375, 400, 425, 450, 475 or 500 mM. Preferably, the sodium chloride is present in the formulation at a concentration of about 125mM. [00067] In some embodiments, the sodium citrate is present in the formulation at a concentration in the range of about 10 mM to 100 mM. For example, the sodium citrate may be present in the formulation at a concentration of about 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 95 or 100 mM. Preferably, the sodium citrate is present in the formulation at a concentration in the range of about 25 to 50 mM. [00068] In some embodiments, the formulation comprises more than one salt. In some embodiments, the formulation comprises sodium acetate trihydrate at a concentration in the range of about 25 mm to 100 mm and sodium chloride at a concentration in the range of about 150 mm to 500 mm. [00069] Preferably, the formulation comprises about 25 mM sodium acetate trihydrate and about 150 mM sodium chloride. [00070] In some embodiments, the formulation comprises one or more polyols as a bulking agent and/or stabilizing excipients. Polyols can include, for example, trehalose, mannitol, maltose, lactose, sucrose, sorbitol, or glycerol. In some embodiments, the polyol is
Attorney Docket No. TIZI-035/001WO 322161-2568 present in the formulation at a concentration in the range of about 0.1% to 50% or 5% to 25%. For example, the polyol may be present in the formulation at a concentration of about 1, 2, 3, 4, 5, 10, 15, 20, 25, 30, 35, 40, 45 or 50% [00071] The formulation may also comprise one or more excipients and/or surfactants to suppress or otherwise reduce antibody aggregation. Examples of surfactants that may be used to reduce antibody aggregation include Polysorbate 20 or Polysorbate 80. In some embodiments, the Polysorbate 20 or Polysorbate 80 is present at a concentration in the range of about 0.01 to 1 % or about 0.01 to 0.05%. For example, the Polysorbate 20 or Polysorbate 80 is at a concentration of about 0.01.0.02, 0.03, 0.04, 0.05, 0.06, 0.07.0.08, 0.09, 0.1, 0.2, 0.3.0.4, 0.5, 0.6, 0.7, 0.8.0.9, or 1.0 %. [00072] Preferably, the surfactant is Polysorbate 80 present in the formulation at a concentration in the range of about 0.01 to 0.05%. More preferably, the Polysorbate 80 is present in the formulation at a concentration of 0.02%. [00073] In some embodiments, the formulation comprises one or more excipients to increase stability. In some embodiments, the excipient to increase stability is human serum albumin. In some embodiments, the human serum albumin is present in the formulation at a concentration in the range of about 1 mg to about 5 mg. [00074] In some embodiments, the formulation comprises magnesium stearate (Mg stearate), an amino acid, or both Mg-stearate and an amino acid. Suitable amino acids include for example, leucine, arginine, histidine, or combinations thereof. [00075] The formulation may comprise additional suitable excipients. In some embodiments the one or more additional excipients is low moisture microcrystalline cellulose, such as Avicel, polyethylene glycols (PEG), or a starch. [00076] Further examples of pharmaceutically acceptable carriers and excipients useful for the formulations of the present disclosure include, but are not limited to binders, fillers, disintegrants, lubricants, anti-microbial agents, antioxidant, and coating agents such as: BINDERS: corn starch, potato starch, other starches, gelatin, natural and synthetic gums such as acacia, xanthan, sodium alginate, alginic acid, other alginates, powdered tragacanth, guar gum, cellulose and its derivatives (e.g., ethyl cellulose, cellulose acetate, carboxymethyl cellulose calcium, sodium carboxymethyl cellulose), polyvinyl pyrrolidone (e.g., povidone, crospovidone, copovidone, etc.), methyl cellulose, Methocel, pre-gelatinized starch (e.g., STARCH 1500® and STARCH 1500 LM®, sold by Colorcon, Ltd.), hydroxypropyl methyl cellulose, microcrystalline cellulose (FMC Corporation, Marcus Hook, PA, USA), Emdex, Plasdone, or mixtures thereof, FILLERS: talc, calcium carbonate (e.g., granules or powder),
Attorney Docket No. TIZI-035/001WO 322161-2568 dibasic calcium phosphate, tribasic calcium phosphate, calcium sulfate (e.g., granules or powder), microcrystalline cellulose, powdered cellulose, dextrates, kaolin, mannitol, silicic acid, sorbitol, starch, pre-gelatinized starch, dextrose, fructose, honey, lactose anhydrate, lactose monohydrate, lactose and aspartame, lactose and cellulose, lactose and microcrystalline cellulose, maltodextrin, maltose, mannitol, microcrystalline cellulose & guar gum, molasses, sucrose, or mixtures thereof, DISINTEGRANTS: agar-agar, alginic acid, calcium carbonate, microcrystalline cellulose, croscarmellose sodium, crospovidone, polacrilin potassium, sodium starch glycolate, (such as Explotab), potato or tapioca starch, other starches, pre-gelatinized starch, clays, other algins, other celluloses, gums (like gellan), low-substituted hydroxypropyl cellulose, ployplasdone, or mixtures thereof, LUBRICANTS: calcium stearate, magnesium stearate, mineral oil, light mineral oil, glycerin, sorbitol, mannitol, polyethylene glycol, other glycols, compritol, stearic acid, sodium lauryl sulfate, sodium stearyl fumarate, (such as Pruv), vegetable based fatty acids lubricant, talc, hydrogenated vegetable oil (e.g., peanut oil, cottonseed oil, sunflower oil, sesame oil, olive oil, corn oil and soybean oil), zinc stearate, ethyl oleate, ethyl laurate, agar, syloid silica gel (AEROSIL 200, W.R. Grace Co., Baltimore, MD USA), a coagulated aerosol of synthetic silica (Deaussa Co., Piano, TX USA), a pyrogenic silicon dioxide (CAB-O-SIL, Cabot Co., Boston, MA USA), or mixtures thereof, ANTI-CAKING AGENTS: calcium silicate, magnesium silicate, silicon dioxide, colloidal silicon dioxide, talc, or mixtures thereof, ANTIMICROBIAL AGENTS: benzalkonium chloride, benzethonium chloride, benzoic acid, benzyl alcohol, butyl paraben, cetylpyridinium chloride, cresol, chlorobutanol, dehydroacetic acid, ethylparaben, methylparaben, phenol, phenylethyl alcohol, phenoxyethanol, phenylmercuric acetate, phenylmercuric nitrate, potassium sorbate, propylparaben, sodium benzoate, sodium dehydroacetate, sodium propionate, sorbic acid, thimersol, thymo, or mixtures thereof, ANTOXIDANTS: ascorbic acid, BHA, BHT, EDTA, or mixture thereof, and COATING AGENTS: sodium carboxymethyl cellulose, cellulose acetate phthalate, ethylcellulose, gelatin, pharmaceutical glaze, hydroxypropyl cellulose, hydroxypropyl methylcellulose (hypromellose), hydroxypropyl methyl cellulose phthalate, methylcellulose, polyethylene glycol, polyvinyl acetate phthalate, shellac, sucrose, titanium dioxide, carnauba wax, microcrystalline wax, gellan gum, maltodextrin, methacrylates, microcrystalline cellulose and carrageenan or mixtures thereof. [00077] The formulation can also comprise other excipients and categories thereof including but not limited to Pluronic®, Poloxamers (such as Lutrol® and Poloxamer 188), ascorbic acid, glutathione, protease inhibitors (e.g. soybean trypsin inhibitor, organic acids),
Attorney Docket No. TIZI-035/001WO 322161-2568 pH lowering agents, creams and lotions (like maltodextrin and carrageenans); materials for chewable tablets (like dextrose, fructose, lactose monohydrate, lactose and aspartame, lactose and cellulose, maltodextrin, maltose, mannitol, microcrystalline cellulose and guar gum, sorbitol crystalline); parenterals (like mannitol and povidone); plasticizers (like dibutyl sebacate, plasticizers for coatings, polyvinylacetate phthalate); powder lubricants (like glyceryl behenate); soft gelatin capsules (like sorbitol special solution); spheres for coating (like sugar spheres); spheronization agents (like glyceryl behenate and microcrystalline cellulose); suspending/gelling agents (like carrageenan, gellan gum, mannitol, microcrystalline cellulose, povidone, sodium starch glycolate, xanthan gum); sweeteners (like aspartame, aspartame and lactose, dextrose, fructose, honey, maltodextrin, maltose, mannitol, molasses, sorbitol crystalline, sorbitol special solution, sucrose); wet granulation agents (like calcium carbonate, lactose anhydrous, lactose monohydrate, maltodextrin, mannitol, microcrystalline cellulose, povidone, starch), caramel, carboxymethylcellulose sodium, cherry cream flavor and cherry flavor, citric acid anhydrous, citric acid, confectioner's sugar, D&C Red No.33, D&C Yellow #10 Aluminum Lake, disodium edetate, ethyl alcohol 15%, FD&C Yellow No.6 aluminum lake, FD&C Blue # 1 Aluminum Lake, FD&C Blue No.1, FD&C blue no.2 aluminum lake, FD&C Green No.3, FD&C Red No.40, FD&C Yellow No. 6 Aluminum Lake, FD&C Yellow No.6, FD&C Yellow No.10, glycerol palmitostearate, glyceryl monostearate, indigo carmine, lecithin, manitol, methyl and propyl parabens, mono ammonium glycyrrhizinate, natural and artificial orange flavor, pharmaceutical glaze, poloxamer 188, Polydextrose, polyvidone, pregelatinized corn starch, pregelatinized starch, red iron oxide, saccharin sodium, sodium carboxymethyl ether, sodium chloride, sodium citrate, sodium phosphate, strawberry flavor, synthetic black iron oxide, synthetic red iron oxide, titanium dioxide, and white wax. [00078] In some aspects, the foralumab formulation provided herein is a nasal formulation. [00079] In some embodiments the formulation for nasal delivery comprises 0.25 mg/ ml foralumab, 3.4 mg/mL sodium acetate, 0.20 mg/ml polysorbate 80 and 7.31 mg/ ml sodium chloride. [00080] In other embodiments the formulation for nasal delivery comprises 0.5 mg/ ml foralumab, 3.4 mg/mL sodium acetate, 0.20 mg/ml polysorbate 80 and 7.31 mg/ ml sodium chloride. [00081] In some embodiments, the osmolality of the formulation is about 800-950 (e.g., about 825-925) mOsm/kg.
Attorney Docket No. TIZI-035/001WO 322161-2568 [00082] In some embodiments, the nasal formulation is an aerosol formulation. In some embodiments, the nasal formulation is suitable for once daily administrations. In some embodiments, the nasal formulation provides for aerosol of the antibody at a dosage in the range of about 10 μg to 100 μg per single administration. In some embodiments, the nasal formulation provides for aerosol of the antibody at a dosage of 25 μg 50 μg per single administration. In some embodiments, the single administration is administered to one nostril or, alternatively, split between both nostrils. [00083] In some embodiments, the average droplet size of the delivered formulation is between 10 μm and 250 μm. For example, the droplet size may be between 10 μm and 100 μm, or between 25 μm and 250 μm. [00084] In some embodiments, the nasal formulation is suitable for storage at about 2 °C to about 4 °C. In some embodiments, the nasal formulation is stored in a sealed vial or other suitable container. In some embodiments, the nasal formulation is stored in a sealed vial or other suitable container at about 2 °C to about 4 °C. [00085] GLP-1 Agonists [00086] Glucagon-like peptide-1 (GLP-1) receptor agonists (also referred to as GLP-1 agonists or GLP-1 analogs) are medications that help lower blood sugar levels and promote weight loss. They mimic the GLP-1 hormone and increase insulin release from the pancreas., leading to lower blood sure and increased satiety). GLP-1 agonists are used to control type 2 diabetes as well as treat obesity and improve obesity-related complications. [00087] Diabetes drugs in the GLP-1 agonists class are generally taken by a shot (injection) given daily or weekly. Some oral formulations are available. [00088] GLP-1 agonists that are currently approved for clinical use are summarized in [00089] Table 1. Table 1: Approved GLP-1 Agonists
Attorney Docket No. TIZI-035/001WO 322161-2568
[00090] GLP-1 agonists that are currently in clinical development are summarized in Table 2. GLP-1 agonists and other obesity drugs are further described in Melson et al., Int J Obes (2024). https://doi.org/10.1038/s41366-024-01473-y.
Attorney Docket No. TIZI-035/001WO 322161-2568 - 3 - 3
D -
y e t s i a h - - - - - – e e 2 3 e d i P 3 e 2 e 3 eD
2 e 2 e 1
L G
L L L I L m G G G G G A
L G
C G
L G
C G
L G
I G
L G
L G
C G
L G
C G
L G
I G
n a D O D ,
O W O W W W W W D
e c y B W W
n o l h t O
, O
, O O O O O C , , , , , , O , O , , n o P P S C S C S C S C S C S O P C S C S C S m g
g g / g g g g g m m
g g m
g m 5
2 m 4 m
– m–
0 m – m 0 A
– 5 5 46 1 2 2 3 m 6 m 9
– 2 4 4 1 42 5 1 1 2 A N
. 7 . . . 2 00 – 0 . . N *
e di n e * n e t o u r di t e l p d a e d i t e e d o r -
d it 3 g il u l i t m e u di
t i
t pi l g e u 3 a g r g a a p S i d o u d ur
t g p d o e i v 1 m
of m
e z r r g v r z a a t u n n i di t m G e
S r O
e S i T a C
u S M
e R
a D
f E u d e P M A
Attorney Docket No. TIZI-035/001WO 322161-2568
) s ( n oi t a c i d n I n o i t e l p o c y n a p o C
t
c a L G
Y P L G
L t G
c h o A
n i g a L G
L P G
I P P P o P G
L G
I G
L G
I G
L G
I G
L m G A
Y P g a L G
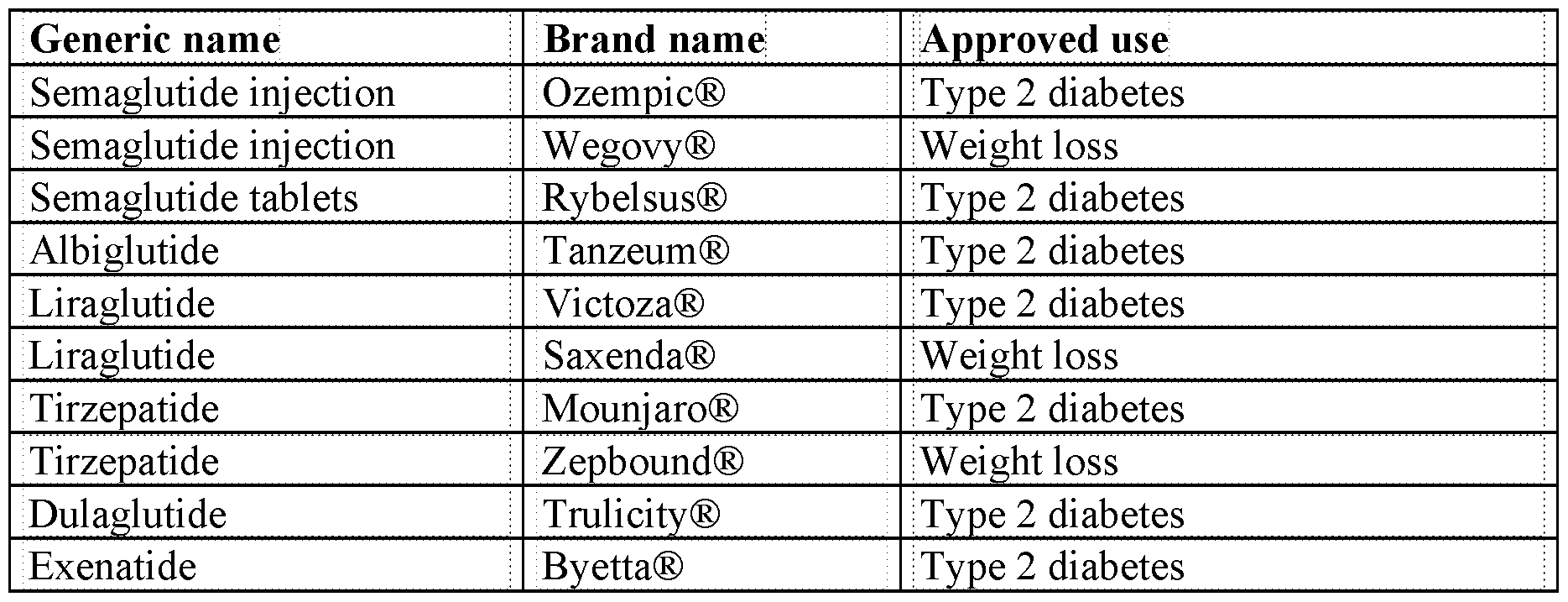
I G 2 n
y o r s k 4 e y r + ) y c d e y c d e i e v e W
e v s - a b aW D
n e t a t W
n e t a W D W W t
a e r
t , w O s
C 4 , e k S o t C , e S
V I e m i m u O w
B ( r , O g
C , S O , u s O P
O q P er
t f o , n C , u t s O S
O q P er
t f o , O n
C , O O S
O , P C , S C S g g e g g g s m
o 2 m m
g k –
4 6 / g – 1 m
g m 4
+ . 2 4 3 A A A A
m 0 A m +
2 N N
– 5 21 – 1 01 A D
1 – 0 . N N N N -
e 5 d 6 i e b t
d a e i m
di t -
4 n it 7 8 - e 5 di t 1 e 1 u 0 + l t g
m a u l u g r u l 6 5 3 5 3 9 8 e r 4 6 1 d n u l 1 2 i g a g a 9 a C 5 9 7 7 0 - 8 0 3 c y C) 0 a g a 5 1 N
7 m
p a m
i m
- 2 K
2 K O - mN
1 1 C N
2 6m M N N
8 1 e S D
B + e S T C V V
C S T C A
N ( 1 0 N
5 1 e S H
Attorney Docket No. TIZI-035/001WO 322161-2568 )
s ( n oi t a c i d n I s l a i r T
l a c i
n
o i i -
l v t c y P e L V I C
o g n u f n G s d i , ,
l k
t s a i r d n y e t i d n o o c t e c c i
i o O l
n g a P e l p p xm
e o o t b r a
t h r , s c o t u E o c a d e D
p o e e . c n y- l
mK a
c H
Ce r a t 3 7 i
S , u e A c 4 1 y
t n u A s a R b u 0-
e Mes
, a s 4 a c p
a , n o i e o C
2 i d r n S , 0- m m o
r t a
c e p 6 a a Y
6 r
v i l
p Y
3 C
h P f n c o i
e 1 i t el e 4 t s di
s/ c o t e
j a e e t p 8 3 t
v i e p 0 1 f e s t c . o d e d e u Y r
0 t Y 1 / m
s v i r t e a i s c
b P, g o n r n s e o o . i a r o h
p s s a A S g a o c d / / c n e oi t ht - i
n O, s u l . : g e l s M
c a woi e
t r c i t i b p t r G
al
t h u
n h C
i l
u f t
r a . a G v ) 4 - i y
t i i
a f s y n
d o , e e a d t 2 o 0 i
n o s e tr t i a
c i s o t p n 2 ( mi t d a b o e l h o e a b A p t a s e b A
r t r s o f F a t e O , y r l o d A O s
l E e a i p a F ml p u c N J i , t n I s
r t H o
3 , D s
L c s a p o y e
S v r l t k , o
e .
l e a D
e s a t A o i n t e h e p b a M
d i
, s r i a
l w u -
e c n o d e d it
c s t e 2 i t V n i n s t o l e e l p e a p p e C n W
M h , e e d O : e m a m y t o
o s D
t a a e n e , yl
c r N
C * 2 T et s s i p i u d e d a d o S
Attorney Docket No. TIZI-035/001WO 322161-2568 [00091] GLP-1 agonists have also been shown to improve obesity-related complications such as liver function markers, fat accumulation in the liver, fibrosis, fatty liver diseases and inflammation. Cardiovascular protection and renal protection have also been reported with GLP- 1 agonists. Without wishing to be bound by theory, it is hypothesized that these effects will be augmented by co-administration of a GLP-1 agonist with foralumab, since foralumab is able to reprogram T cells to promote immune tolerance and tissue homeostasis. SGLT-2 Inhibitors [00092] Sodium-glucose cotransporter-2 (SGLT2) inhibitors are a class of oral prescription medicines that are FDA-approved for use with diet and exercise to lower blood sugar in adults with type 2 diabetes. [00093] Some SGLT-2 inhibitors are also FDA-approved for use in people with chronic kidney disease (CKD) and/or heart failure to lower the risk of heart attack, stroke, and/or heart failure flare-ups, including in people who do not have diabetes. Some SGLT-2 inhibitors are FDA approved to help slow the progression of kidney disease. [00094] SGLT-2 inhibitors include but not limited to Brenzavvy™ (bexaglifloxin), Invokana® (canagliflozin), Farxiga® (dapagliflozin), Jardiance® (empagliflozin), and Steglatro® (ertugliflozin). Methods of Treatment [00095] Provided herein are methods of treatment comprising the administration of foralumab together with either a GLP-1 agonist (or dual GLP-1/GIP receptor agonist) or a SGLT-2 inhibitor. In some embodiments, the methods are used to treat, prevent, or reduce a comorbidity associated with chronic inflammation such as obesity, Type 1 Diabetes (T1D), Type 2 Diabetes (T2D), chronic kidney disease, metabolic syndrome, and cardiovascular risk. By cardiac risk it is meant to treat, prevent, or reduce heart failure, to lower the risk of heart attack, stroke, and/or heart failure flare-ups. In particular embodiments the methods and formulations provided herein lowers the risk of major adverse cardiovascular events (MACE).The therapeutic formulations may be administered to a subject suffering from chronic inflammation, diabetes (type 1 or type 1), a body mass index of 25 or greater, at risk of developing diabetes, does not have diabetes, has kidney disease (e.g. chronic kidney disease), at risk of a heart attack or stroke
Attorney Docket No. TIZI-035/001WO 322161-2568 or have previously had a heart attack or stroke. In some aspects, the subject has heart failure, such as heart failure with preserved injection fraction. Methods of Treating Obesity-Related Complications [00096] In another aspect, provided herein is a method of treating an obesity-related complication in a subject in need thereof, comprising administering to the subject a GLP-1 agonist (or dual GLP-1/GIP receptor agonist) and foralumab. Examples of obesity-related complications include inflammation, abnormalities in metabolism, and liver disease. The outcome of a method of treatment may be assessed at any suitable time, for example, 1 weeks, 2 weeks, 3 weeks, one month, 2 months, or 3 months after the beginning of the treatment. Inflammation [00097] In some embodiments, the obesity-related complication is inflammation. The inflammation may be liver inflammation, adipose tissue inflammation, or systemic inflammation. In some embodiments, the obesity-related complication treated in accordance with a method described herein is adipose tissue inflammation. [00098] Systemic inflammation can be determined using markers such as TNFα, IL-1β or keratinocyte chemoattractant/growth-regulated oncogene (KC/GRO). In some embodiments, a method of treating an obesity-related complication described herein results in a decrease in blood levels of TNFα of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to blood levels of TNFα prior to treatment. In some embodiments, a method of treating an obesity-related complication described herein results in a decrease in blood levels of IL-1β of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to blood levels of IL-1β prior to treatment. In some embodiments, a method of treating an obesity-related complication described herein results in a decrease in blood levels of KC/GRO of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to blood levels of KC/GROP prior to treatment. [00099] Adipose tissue inflammation can be measured using a variety of markers, for example, IL-6 or IL-10, or it can be determined by histology. Additional, adipose inflammation
Attorney Docket No. TIZI-035/001WO 322161-2568 can be determined by gene expression analysis. or by measuring the amount of adipose tissue macrophages. In some embodiments, a method of treating an obesity-related complication described herein results in a decrease in IL-6 expression in adipose tissue of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the IL-6 expression in adipose tissue prior to treatment. In some embodiments, a method of treating an obesity-related complication described herein results in a decrease in IL-10 expression in adipose tissue of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the IL-10 expression in adipose tissue prior to treatment. [000100] In some embodiments, the obesity-related complication treated in accordance with a method described herein is liver inflammation. Liver inflammation may be determined using markers such as ALT, AST or BUN. Liver inflammation can also be measured by determining the numbers of inflammatory cells such as neutrophils, pro-inflammatory Tim4- monocytes- derived macrophages or F4-80-positive Kupffer cells in liver tissue. Liver inflammation may also be determined by gene expression analysis. [000101] In some embodiments, a method of treating an obesity-related complication described herein results in a decrease blood levels of ALT of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to blood levels of ALT prior to treatment. In some embodiments, a method of treating an obesity-related complication described herein results in a decrease blood levels of AST of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to blood levels of AST prior to treatment. In some embodiments, a method of treating an obesity-related complication described herein results in a decrease blood levels of BUN of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to blood levels of BUN prior to treatment. [000102] In some embodiments, a method of treating an obesity-related complication described herein results in a decrease in the number of neutrophils in liver tissue of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least
Attorney Docket No. TIZI-035/001WO 322161-2568 about 60%, at least about 70%, at least about 80% or at least about 90% compared to the number of neutrophils in liver tissue prior to treatment. In some embodiments, a method of treating an obesity-related complication described herein results in a decrease Tim4- monocytes-derived macrophages in liver tissue of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the levels of Tim4- monocytes-derived macrophages prior to treatment. In some embodiments, a method of treating an obesity-related complication described herein results in a decrease in F4/80-positive cells of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to levels prior to treatment. Liver Damage [000103] In some embodiments, the obesity-related complication treated in accordance with a method described herein is liver damage. Liver damage may be assessed using any suitable method known in the art or described herein. [000104] Lipid accumulation in the liver may be measured by staining liver tissue with O Red Oil and determining the area of the tissue that is stained. In some embodiments, a method of treating an obesity-related complication described herein results in a decrease in lipid accumulation in the liver of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the levels of lipid accumulation after treatment with the GLP-1 agonist prior to treatment. [000105] Liver damage can also be determined by elevated blood levels of liver enzymes such as ALT and AST. In some embodiments, a method of treating an obesity-related complication described herein results in a decrease blood levels of ALT of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to blood levels of ALT prior to treatment. In some embodiments, a method of treating an obesity-related complication described herein results in a decrease blood levels of AST of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to blood levels of AST prior to treatment. In some embodiments, a method of treating an obesity-related complication described
Attorney Docket No. TIZI-035/001WO 322161-2568 herein results in a decrease blood levels of BUN of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to blood levels of BUN prior to treatment. [000106] Liver damage in the form of lesions may also be determined using imaging such as PET or MRI to measure the contrast to noise ratio (CNR). In some embodiments, a method of treating an obesity-related complication described herein results in a decrease in the CNR of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the CNR prior to treatment. [000107] Another marker of liver damage is lactate dehydrogenase (LDH). LDH is associated with tissue damage and may be used to determine liver damage. In some embodiments, a method of treating an obesity-related complication described herein results in a decrease in blood levels of LDH of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the blood levels of LDH prior to treatment. Fibrosis [000108] In some embodiments, the obesity-related complication treated in accordance with a method described herein is fibrosis. Fibrosis may be determined by staining liver tissue with Sirius Red and assigning a histopathology score based on the stain that measures fibrosis. In some embodiments, a method of treating an obesity-related complication described herein results in a decrease in liver fibrosis as measured by histopathological score of at least about 10%, at least about 20%, at least about 30%, at least about 40% or at least about 50% compared to the histopathological score prior to treatment. [000109] Liver fibrosis may also be measured by stellate cell activation in the liver. Stellate cell activation can be measured using staining liver tissue for alpha-smooth muscle actin (SMAα). In some embodiments, a method of treating an obesity-related complication described herein results in a decrease in liver stellate cell activation as measured by SMAα of at least about 10%, at least about 20%, at least about 30%, at least about 40% or at least about 50% compared to the SMAα level prior to treatment. [000110] Alternatively, or additionally, liver fibrosis can be determined using MRI scans to measure the T1 relaxation time of the liver. A shorter T1 relaxation time is associated with
Attorney Docket No. TIZI-035/001WO 322161-2568 higher levels of fibrosis. In some embodiments, a method of treating an obesity-related complication described herein results in an increase in T1 of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the T1 prior to treatment. Metabolic Abnormalities [000111] In some embodiments, the obesity-related complication treated in accordance with a method described herein is a systemic metabolism abnormality. Examples of systemic metabolism abnormalities include, for example, changes in liver function or fat metabolism. In some embodiments, the systemic metabolism abnormality is an increase in the blood levels of cholesterol, triglycerides, aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), amylase, and/or lipases. [000112] In some embodiments, a method of treating an obesity-related complication described herein results in a decrease in blood levels of AST of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the blood levels of AST prior to treatment. In some embodiments, a method of treating an obesity-related complication described herein results in a decrease in blood levels of ALT of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the blood levels of ALT prior to treatment. In some embodiments a method of treating an obesity-related complication described herein results in a decrease in blood levels of BUN of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the blood levels of BUN prior to treatment. In some embodiments, a method of treating an obesity-related complication described herein results in a decrease in blood levels of total cholesterol of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the blood levels of total cholesterol prior to treatment. In some embodiments, a method of treating an obesity-related complication described herein results in a decrease in blood levels of triglycerides of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at
Attorney Docket No. TIZI-035/001WO 322161-2568 least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the blood levels of triglycerides prior to treatment. Methods of Increasing Efficacy of GLP-1 Agonists [000113] In another aspect, provided herein is a method of increasing the efficacy of a GLP-1 agonist in a subject, comprising administering to the subject a GLP-1 agonist (or dual GLP-1/GIP receptor agonist) and foralumab. The increase in efficacy can be, for example, an increase in the improvement in metabolism, a decrease in liver disease markers, or an increase in liver regeneration. The increase in efficacy may be assessed at any suitable time, for example, 1 weeks, 2 weeks, 3 weeks, one month, 2 months, or 3 months after the beginning of the treatment. The improvement in efficacy may also be an improvement in one or more adverse effects of a GLP-1 agonist, including adverse effects that only appear upon discontinuation of the administration of the GLP-1 agonist. Without wishing to be bound by theory, it is believed that continues administration of foralumab after discontinuation of the GLP-1 agonist may ameliorate such adverse effects that only become apparent after discontinuation of the GLP-1 agonist. Inflammation [000114] In some embodiments, the improvement in efficacy is a decrease in inflammation. This may be a decrease in liver inflammation, adipose tissue inflammation, or systemic inflammation, or a combination of these. [000115] As described above, systemic inflammation can be determined using markers such as TNFα, IL-1β or KC/GRO. In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in a decrease in blood levels of TNFα of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to blood levels of TNFα after treatment with the GLP-1 agonist in the absence of foralumab. In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in a decrease in blood levels of IL-1β of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to blood levels of IL-1β after treatment with the GLP-1 agonist in the absence of foralumab. In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in a decrease in blood levels of KC/GRO of at least about
Attorney Docket No. TIZI-035/001WO 322161-2568 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to blood levels of KC/GROP after treatment with the GLP-1 agonist in the absence of foralumab. [000116] In some embodiments, the improvement in efficacy is a decrease in liver inflammation. As described above, liver inflammation may be determined using markers such as ALT, AST or BUN, or by measuring the levels of inflammatory cells such as neutrophils, pro- inflammatory Tim4- monocytes-derived macrophages or F4-80-positive Kupffer cells. Additionally, liver inflammation may be measured using gene expression analysis. [000117] In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in a decrease blood levels of ALT of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to blood levels of ALT after treatment with the GLP-1 agonist in the absence of foralumab. In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in a decrease blood levels of AST of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to blood levels of AST after treatment with the GLP-1 agonist in the absence of foralumab. In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in a decrease blood levels of BUN of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to blood levels of BUN after treatment with the GLP-1 agonist in the absence of foralumab. [000118] In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in a decrease in the number of neutrophils in liver tissue of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the number of neutrophils in liver tissue after treatment with the GLP-1 agonist in the absence of foralumab. In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in a decrease Tim4- monocytes-derived macrophages in liver tissue of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the levels of
Attorney Docket No. TIZI-035/001WO 322161-2568 Tim4- monocytes-derived macrophages after treatment with the GLP-1 agonist in the absence of foralumab. In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in a decrease in F4/80-positive cells of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to levels after treatment with the GLP-1 agonist in the absence of foralumab. [000119] As described above, adipose tissue inflammation can be measured using markers such as IL-6 or IL-10, or it can be determined by histology. Additional, adipose inflammation can be determined by gene expression analysis or by measuring the amount of adipose tissue macrophages. In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in a decrease in IL-6 expression in adipose tissue of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the IL-6 expression in adipose tissue after treatment with the GLP-1 agonist in the absence of foralumab. In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in a decrease in IL-10 expression in adipose tissue of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the IL-10 expression in adipose tissue after treatment with the GLP-1 agonist in the absence of foralumab. [000120] In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in a decrease in blood levels of LDH of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the blood levels of LDH after treatment with the GLP-1 agonist in the absence of foralumab. Liver Damage [000121] In some embodiments, the improvement in efficacy is a decrease in liver damage. As described above, liver damage may be determined by measuring lipid accumulation in the liver, blood levels of liver enzymes such as ALT and AST or LDH, or using imaging such as PET or MRI to detect lesions and relaxation time T1. [000122] In some embodiments, a method of increasing the efficacy of a GLP-1 agonist an obesity-related complication described herein results in a decrease in the CNR of at least about
Attorney Docket No. TIZI-035/001WO 322161-2568 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the CNR after treatment with the GLP-1 agonist in the absence of foralumab. Fibrosis [000123] In some embodiments, the improvement in efficacy is a decrease in fibrosis. As described above, fibrosis may be determined by staining liver tissue with Sirius Red or by measuring stellate cell activation as determined by SMAα staining. [000124] In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in a decrease in liver fibrosis as measured by histopathological score of at least about 10%, at least about 20%, at least about 30%, at least about 40% or at least about 50% compared to the histopathological score after treatment with the GLP-1 agonist alone. In some embodiments, a method of treating described herein results in a decrease in liver stellate cell activation as measured by SMAα of at least about 10%, at least about 20%, at least about 30%, at least about 40% or at least about 50% compared to the SMAα level after treatment with the GLP-1 agonist in the absence of foralumab. In some embodiments, a method o of increasing the efficacy of a GLP-1 agonist described herein results in an increase in T1 of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the T1 after treatment with the GLP-1 agonist in the absence of foralumab. Homeostasis [000125] In some embodiments, the improvement in efficacy is an improvement in liver homeostasis. Liver homeostasis includes various metabolic pathways, such as lipid metabolism, glucose metabolism and bile acid metabolism. These pathways can be analyzed using genetic biomarkers. Liver homeostasis can also be determined by histopathological analysis and assigning a histopathological score including inflammatory infiltrate (portal, lobular, pericentral); steatosis (macro- and Micro-vesicular); hepatocellular injury (ballooning, Mallory body, acidophilic body, and necrosis); bile duct injury; fibrosis (based on Sirius red staining) including portal, pericellular, bridging, and cirrhosis. Generally, healthier livers are smaller and contain less fat compared to diseased livers. [000126] In some embodiments, the improvement in efficacy is an improvement in adipose homeostasis. Adipose homeostasis includes factors such as inflammation and lipid metabolism,
Attorney Docket No. TIZI-035/001WO 322161-2568 each of which can be determined by gene expression analysis. Thus, inflammation can be measured by gene expression of, for example, Ccl7, Ccl5, Nfil3, Sod3, Mrc1, Tlr2, Ilrun, Chuk, Cd163, Ccl9, Cxcl12, Jak1, Nfkbia, Stat3, Traf2, Nrp1, Ifit2, and Cd163 are markers of adipose inflammation. [000127] In some embodiments, the improvement in efficacy is a decrease in lipid accumulation in the liver. Lipid accumulation in the liver may be measured by staining liver tissue with O Red Oil and determining the area of the tissue that is stained. In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in a decrease in lipid accumulation in the liver of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the levels of lipid accumulation after treatment with the GLP-1 agonist in the absence of foralumab. [000128] In some embodiments, the improvement in efficacy is an increase in brown adipose tissue thermogenesis. Brown adipose tissue thermogenesis can be determined by staining tissue for markers such as Uncoupling protein 1 (UCP-1). In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in an increase in UCP-1 expression of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the levels of UCP-1 expression after treatment with the GLP-1 agonist in the absence of foralumab. [000129] In some embodiments, the improvement is an improvement in a biomarker of systemic lipid metabolism. Examples of biomarkers of metabolism include AST, ALT, BUN, cholesterol, or triglycerides. In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in a decrease in blood levels of AST of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the blood levels of AST after treatment with the GLP-1 agonist in the absence of foralumab. In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in a decrease in blood levels of ALT of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the blood levels of ALT after treatment with the GLP-1
Attorney Docket No. TIZI-035/001WO 322161-2568 agonist alone. In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in a decrease in blood levels of BUN of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the blood levels of BUN after treatment with the GLP-1 agonist alone. In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in a decrease in blood levels of total cholesterol of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the blood levels of total cholesterol after treatment with the GLP-1 agonist alone. In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in a decrease in blood levels of triglycerides of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to the blood levels of triglycerides after treatment with the GLP-1 agonist alone. [000130] In some embodiments, the improvement in efficacy is an increase in liver regeneration. Liver regeneration can be determined histologically or by measuring biomarkers of extracellular matrix (ECM) organization. Biomarkers of ECM organization include genes such as Acta2, Angptl8, Clnd1, Col1a1, Col4a1, Col4a2, Col6a1, Col6a2, Col6a3, Cox19, Creg1, Ecm1, Flnb, Fn1, Itgax, Itgb1bp1, Lamc1, Mmp2, Mmp15, Plod1, Timp2, Vcam1, Zfx, and Zyx. Downregulation of these markers is associated with decreased fibrosis in the liver. In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in an increase in liver regeneration of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared the levels of liver regeneration observed prior to treatment. [000131] Alternatively, or additional, liver regeneration can be measured by determining the amount of Tim4-positive Kupffer cells in the liver. In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in an increase in Tim4- positive cells of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to levels after treatment with the GLP-1 agonist in the absence of foralumab.
Attorney Docket No. TIZI-035/001WO 322161-2568 [000132] In some embodiments, a method described herein results in comparable weight loss to treatment with the GLP-1 agonist alone. [000133] Sarcopenia [000134] In some embodiments, the improvement is reduction in sarcopenia. Sarcopenia Is a condition characterized by loss of muscle mass, strength and function. It has been reported that GLP-1 agonists may lead to sarcopenia in patients due to the rapid weight loss. In some embodiments, a method of increasing the efficacy of a GLP-1 agonist described herein results in a decrease in sarcopenia of at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80% or at least about 90% compared to sarcopenia after treatment with the GLP-1 agonist in the absence of foralumab. Sarcopenia may be assesses using any suitable method known in the art, for example, imaging such as MRI or CT, as well as functional endpoints such as muscle strength and physical performance (see, e.g., Guttikonda et al., Clin Liver Dis (Hoboken).2021 Oct 27;18(4):189–192). Administration and Doses [000135] The methods disclosed herein provide for the administration of a GLP-1 agonist (or dual GLP-1/GIP receptor agonist) in combination with foralumab. The doses, frequency and route of administration of the GLP-1 agonist and foralumab in the methods described herein may be varied based on factors such as patient characteristics, disease severity and tolerability. GLP-1 Agonist [000136] The GLP-1 agonist (or dual GLP-1/GIP receptor agonist) may be administered at the dosage approved for the treatment of obesity or type 2 diabetes. This dosage may be found, for example, in the Prescribing Information (also referred to as “drug label”) issued by the US Food and Drug Administration. [000137] Ozempic® (semaglutide) is generally administered once weekly by subcutaneous injection. The starting dose is generally 0.25 mg once weekly which increases to 0.5 mg once weekly after 4 weeks. The dose may be further increased to 1 mg once weekly after at least 4 weeks on the 0.5 mg dose and further to 2 mg once weekly after at least 4 weeks on the 1 mg dosage, if additional glycemic control is needed.
Attorney Docket No. TIZI-035/001WO 322161-2568 [000138] Byetta (exenatide) is generally administered by subcutaneous injection two a day approximately 6 hours or more apart) within 60 minutes prior to meals. The starting dose is generally 5 mcg per dose, which is increased to 10 mcg per dose after one month. [000139] Bydureon and Bydureon BCise (exenatide) are generally administered by subcutaneous injection once every seven days. The dose is generally 2 mg per dose. [000140] Victoza® (liraglutide) is generally administered once daily by subcutaneous injection. The starting dose is generally 0.6 mg once daily, which is increased to 1.2 mg once daily after one week, but can be increased further to 1.8 mg once daily after one week of treatment with the 1.2 mg daily dose if further glycemic control is required. [000141] Saxenda® (liraglutide) is generally administered once daily by subcutaneous administration. The starting dose is generally 0.6 mg per day for one week, which is then increased in weekly intervals until a dose of 3 mg per day is reached. [000142] Adlyxin® (lixisenatide) is generally administered once daily by subcutaneous injection. The starting dose is generally 10 mcg once daily which is increased to 20 mcg once daily after 14 days. [000143] Rybelsus® (semaglutide) is generally administered once daily orally at least 30 minutes before the first food, beverage, or other oral medications of the day with no more than 4 ounces of plain water only. The starting dose is generally 3 mg once daily, which is increased to 7 mg once daily after 30 days on the 3 mg dosage and may be increased to 14 mg once daily if additional glycemic control is needed after at least 30 days on the 7 mg dosage. [000144] Mounjaro® (tirzepatide) is generally administered once weekly by subcutaneous injection. The starting dose generally 2.5 mg once weekly which is increased to 5 mg once weekly after four weeks. The dosage may be increased further in 2.5 mg increments after at least 4 weeks on the current dose to a maximum of 15 mf once weekly, if additional glycemic control is required. [000145] Zepbound ® (tirzepatide) is generally administered once weekly by subcutaneous injection. The starting dosage is generally 2.5 mg once weekly, which is increased to 5 mg once weekly after 4 weeks, and then further increased in 2.5 mg increments after at least 4 weeks on the current dose to a maintenance dose of 5 mg, 10 mg, or 15 mg once weekly, depending on treatment response and tolerability.
Attorney Docket No. TIZI-035/001WO 322161-2568 [000146] Wegovy® (semaglutide) is generally administered once weekly by subcutaneous injection. The starting dose is generally 0.25 mg once weekly for four weeks. The dose is then escalated to 0.5 mg once weekly in weeks 5 through 8, to 1 mg once weekly in weeks 9 through 12, and to 1.7 mg once weekly in weeks 13 through 16. The dose is then maintained at 1.7 mg or 2.4 mg (recommended) once weekly after week 16, depending on treatment response and tolerability. [000147] Tanzeum® (albiglutide) is generally administered once weekly by subcutaneous injection. The starting dose is generally 30 mg once weekly which can be increased to 50 mg once weekly in patients requiring additional glycemic control. [000148] Trulicity® (dulaglutide) is generally administered once weekly by subcutaneous injection. The starting dose is generally 0.75 mg once weekly which can be increased to 1.5 mg once weekly for additional glycemic control. In adult patients, the dose may be further increased in 1.5 mg increments after at least 4 weeks on the current dosage to a maximum recommended dose of 4.5 mg once weekly. [000149] A person of skill in the art will be able to determine the appropriate dose and route and frequency of administration for the GLP-1 agonist used in the methods described herein. In some embodiments, the GLP-1 agonist is administered once weekly. In some embodiments, the GLP-1 agonist is administered by subcutaneous injection. Foralumab [000150] Foralumab is preferably administered nasally. In some embodiments, foralumab is administered once daily. In some embodiments, foralumab is administered three times a week. [000151] In some embodiments, a nasal dose of foralumab is between about 20 μg and about 150 μg. In some embodiments, a nasal dose of foralumab is between about 40 μg and about 100 μg. In some embodiments, a nasal dose of foralumab is between about 45 μg and about 105 μg. In some embodiments, a nasal dose of foralumab is 25 μg, 50 μg, 100 μg, 150 μg, 200 μg or 250 μg. The nasal dose may be divided into both nostrils or may be administered into once nostril. In some embodiments, the nasal dose of foralumab is administered in a volume of about 20 µl to about 200 µl. In some embodiments, the nasal dose of foralumab is administered in a volume of about 40 µl to about 120 µl. In some embodiments, the nasal dose of foralumab is administered in a volume of about 60 µl to about 100 µl. In some embodiments, the nasal dose of foralumab is administered in a volume of about 80 µl to about 120 µl. In some embodiments, the
Attorney Docket No. TIZI-035/001WO 322161-2568 nasal dose of foralumab is administered in a volume of about 100 µl to about 150 µl. In some embodiments, the nasal dose of foralumab is administered in a volume of about 150 µl to about 200 µl. [000152] In some embodiments, foralumab is administered at a dose of 50 μg 3 days a week. In some embodiments, foralumab is administered at a dose of 50 μg 3 days a week for two weeks, followed by a one-week rest, comprising a 3-week cycle, for a total of four cycles. [000153] In some embodiments, foralumab is administered at a dose of 100 μg 3 days a week. In some embodiments, foralumab is administered at a dose of 100 μg 3 days a week. for two weeks, followed by a one-week rest, comprising a 3-week cycle, for a total of four cycles. Schedules of Administration [000154] In some embodiments, foralumab is administered prior to the GLP-1 agonist. In some embodiments, foralumab and the GLP-1 agonist are administered simultaneously. [000155] The administration of foralumab may be started first and the administration of the GLP-1 agonist may be added after an appropriate amount of time. In some embodiments, foralumab is administered in the absence of the GLP-1 agonist for a first period of time followed by concurrent administration of foralumab and the GLP-1 agonist for a second period of time. For example, foralumab may be administered without the GLP-1 agonist for 1, 2, 3, 4, 5, 6, or 7 days, after which the foralumab continues to be administered in combination with the GLP-1 agonist. [000156] Alternatively, the administration of the GLP-1 agonist may be started first and the administration of foralumab is added after an appropriate amount of time. In some embodiments, the GLP-1 agonist is administered in the absence of foralumab for a first period of time followed by concurrent administration of foralumab and the GLP-1 agonist for a second period of time. For example, the GLP-1 agonist may be administered without foralumab for 1, 2, 3, 4, 5, 6, or 7 days, after which the GLP-1 agonist continues to be administered in combination with foralumab. [000157] In some embodiments, after foralumab is administered concurrently with the GLP-1 agonist for the second period of time, foralumab may be administered in the absence of the GLP-1 agonist for a third period of time (the maintenance period). For example, the foralumab may be administered in combination with he GLP-1 agonist for about 1, 2, 3, 4, 5, 6, 7 or 8 weeks or for about 2, 3, 4, 5, 6, 7, 8, 910, 11, 12, 13, 14, 15, 16, 17, or 18 months, or for
Attorney Docket No. TIZI-035/001WO 322161-2568 about 1, 2, 3, 4 or 5 years followed by administration of foralumab for about 1, 2, 3, 4, 5, 6, 7 or 8 weeks or for about 2, 3, 4, 5, 6, 7, 8, 910, 11, 12, 13, 14, 15, 16, 17, or 18 months, or for about 1, 2, 3, 4 or 5 years. In some embodiments, the foralumab is administered for the remainder of the patient’s life. [000158] Without wishing to be bound by theory, it is believed that continued administration of foralumab, even in the absence of the GLP-1 agonist results in sustained weight loss and prevent the regaining of the weight lost due to GLP-1 agonist. Furthermore, it is believed that administration of foralumab after discontinuation of the GLP-1 agonist contributes to a reduction in inflammation, liver damage, sarcopenia and accumulation of ectopic fat, as well as improvements in tissue and cell homeostasis and glucose management. [000159] Administration of an anti-CD3 antibody formulation together with a GLP-1 agonist or SGLT-2 inhibitor to a patient suffering from a comorbidity associated with chronic inflammation is considered successful if any of a variety of laboratory or clinical results is achieved. For example, administration of an anti-CD3 antibody formulation together with a GLP-1 agonist or SGLT-2 inhibitor a to a patient suffering from a comorbidity associated with chronic inflammation is considered successful if one or more of the symptoms associated with the disorder is alleviated, reduced, inhibited, or does not progress to a further, i.e., worse, state. [000160] The anti-CD3 antibody formulations formulation together with a GLP-1 agonist or SGLT-2 inhibitor are administered to a subject that is suffering from, has been diagnosed with, or is predisposed to diabetes, cardiovascular disease, kidney disease, or obesity . The anti- CD3 antibody formulations provided herein are administered at a dosage that is sufficient to alleviate at least one symptom of comorbidity associated with chronic inflammation, to treat a comorbidity associated with chronic inflammation, to prevent comorbidity associated with chronic inflammation. Definitions [000161] Unless otherwise defined, scientific and technical terms used in connection with the present disclosure shall have the meanings that are commonly understood by those of ordinary skill in the art. Further, unless otherwise required by context, singular terms shall include pluralities, and plural terms shall include the singular. Generally, nomenclatures utilized in connection with, and techniques of, cell and tissue culture, molecular biology, and protein and oligo- or polynucleotide chemistry and hybridization described herein are those well-known and
Attorney Docket No. TIZI-035/001WO 322161-2568 commonly used in the art. Standard techniques are used for recombinant DNA, oligonucleotide synthesis, and tissue culture and transformation (e.g., electroporation, lipofection). Enzymatic reactions and purification techniques are performed according to manufacturer's specifications or as commonly accomplished in the art or as described herein. The foregoing techniques and procedures are generally performed according to conventional methods well known in the art and as described in various general and more specific references that are cited and discussed throughout the present specification. See e.g., Sambrook et al. Molecular Cloning: A Laboratory Manual (2d ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. (1989)). The nomenclatures utilized in connection with, and the laboratory procedures and techniques of, analytical chemistry, synthetic organic chemistry, and medicinal and pharmaceutical chemistry described herein are those well-known and commonly used in the art. Standard techniques are used for chemical syntheses, chemical analyses, pharmaceutical preparation, formulation, and delivery, and treatment of patients. [000162] As utilized in accordance with the present disclosure, the following terms, unless otherwise indicated, shall be understood to have the following meanings: [000163] As used herein, the term “antibody” refers to immunoglobulin molecules and immunologically active portions of immunoglobulin (Ig) molecules, i.e., molecules that contain an antigen binding site that specifically binds (immunoreacts with) an antigen. Such antibodies include, but are not limited to, polyclonal, monoclonal, chimeric, single chain, F
ab, F
ab’ and F
(ab')2 fragments, and an F
ab expression library. By “specifically bind” or “immunoreacts with” is meant that the antibody reacts with one or more antigenic determinants of the desired antigen and does not react (i.e., bind) with other polypeptides or binds at much lower affinity (K
d > 10
-6) with other polypeptides. [000164] The basic antibody structural unit is known to comprise a tetramer. Each tetramer is composed of two identical pairs of polypeptide chains, each pair having one “light” (about 25 kDa) and one “heavy” chain (about 50-70 kDa). The amino-terminal portion of each chain includes a variable region of about 100 to 110 or more amino acids primarily responsible for antigen recognition. The carboxy-terminal portion of each chain defines a constant region primarily responsible for effector function. Human light chains are classified as kappa and lambda light chains. Heavy chains are classified as mu, delta, gamma, alpha, or epsilon, and define the antibody's isotype as IgM, IgD, IgA, and IgE, respectively. Within light and heavy
Attorney Docket No. TIZI-035/001WO 322161-2568 chains, the variable and constant regions are joined by a “J” region of about 12 or more amino acids, with the heavy chain also including a “D” region of about 10 more amino acids. See generally, Fundamental Immunology Ch.7 (Paul, W., ea., 2nd ed. Raven Press, N.Y. (1989)). The variable regions of each light/heavy chain pair form the antibody binding site. [000165] The term “monoclonal antibody” (MAb) or “monoclonal antibody composition”, as used herein, refers to a population of antibody molecules that contain only one molecular species of antibody molecule consisting of a unique light chain gene product and a unique heavy chain gene product. In particular, the complementarity determining regions (CDRs) of the monoclonal antibody are identical in all the molecules of the population. MAbs contain an antigen binding site capable of immunoreacting with a particular epitope of the antigen characterized by a unique binding affinity for it. [000166] In general, antibody molecules obtained from humans relate to any of the classes IgG, IgM, IgA, IgE and IgD, which differ from one another by the nature of the heavy chain present in the molecule. Certain classes have subclasses as well, such as IgG1, IgG2, and others. Furthermore, in humans, the light chain may be a kappa chain or a lambda chain. [000167] As used herein, the term “epitope” includes any protein determinant capable of specific binding to an immunoglobulin, a scFv, or a T-cell receptor. The term “epitope” includes any protein determinant capable of specific binding to an immunoglobulin or T-cell receptor. Epitopic determinants usually consist of chemically active surface groupings of molecules such as amino acids or sugar side chains and usually have specific three dimensional structural characteristics, as well as specific charge characteristics. An antibody is said to specifically bind an antigen when the dissociation constant is ≤ 1 μM; preferably ≤ 100 nM and most preferably ≤ 10 nM. [000168] As used herein, the terms “immunological binding” and “immunological binding properties” and “specific binding” refer to the non-covalent interactions of the type which occur between an immunoglobulin molecule and an antigen for which the immunoglobulin is specific. The strength, or affinity of immunological binding interactions can be expressed in terms of the dissociation constant (K
d) of the interaction, wherein a smaller K
d represents a greater affinity. Immunological binding properties of selected polypeptides are quantified using methods well known in the art. One such method entails measuring the rates of antigen-binding site/antigen complex formation and dissociation, wherein those rates depend on the concentrations of the
Attorney Docket No. TIZI-035/001WO 322161-2568 complex partners, the affinity of the interaction, and geometric parameters that equally influence the rate in both directions. Thus, both the “on rate constant” (Kon) and the “off rate constant” (K
off) can be determined by calculation of the concentrations and the actual rates of association and dissociation. (See Nature 361:186-87 (1993)). The ratio of Koff /Kon enables the cancellation of all parameters not related to affinity, and is equal to the dissociation constant Kd. (See, generally, Davies et al. (1990) Annual Rev Biochem 59:439-473). An antibody of the present disclosure is said to specifically bind to a CD3 epitope when the equilibrium binding constant (Kd) is ≤1 μM, preferably ≤ 100 nM, more preferably ≤ 10 nM, and most preferably ≤ 100 pM to about 1 pM, as measured by assays such as radioligand binding assays or similar assays known to those skilled in the art. [000169] Conservative amino acid substitutions refer to the interchangeability of residues having similar side chains. For example, a group of amino acids having aliphatic side chains is glycine, alanine, valine, leucine, and isoleucine; a group of amino acids having aliphatic- hydroxyl side chains is serine and threonine; a group of amino acids having amide- containing side chains is asparagine and glutamine; a group of amino acids having aromatic side chains is phenylalanine, tyrosine, and tryptophan; a group of amino acids having basic side chains is lysine, arginine, and histidine; and a group of amino acids having sulfur- containing side chains is cysteine and methionine. Preferred conservative amino acids substitution groups are: valine- leucine-isoleucine, phenylalanine-tyrosine, lysine-arginine, alanine valine, glutamic- aspartic, and asparagine-glutamine. [000170] As discussed herein, minor variations in the amino acid sequences of antibodies or immunoglobulin molecules are contemplated as being encompassed by the present disclosure, providing that the variations in the amino acid sequence maintain at least 75%, more preferably at least 80%, 90%, 95%, and most preferably 99%. In particular, conservative amino acid replacements are contemplated. Conservative replacements are those that take place within a family of amino acids that are related in their side chains. Genetically encoded amino acids are generally divided into families: (1) acidic amino acids are aspartate, glutamate; (2) basic amino acids are lysine, arginine, histidine; (3) non-polar amino acids are alanine, valine, leucine, isoleucine, proline, phenylalanine, methionine, tryptophan, and (4) uncharged polar amino acids are glycine, asparagine, glutamine, cysteine, serine, threonine, tyrosine. The hydrophilic amino acids include arginine, asparagine, aspartate, glutamine, glutamate, histidine, lysine, serine, and
Attorney Docket No. TIZI-035/001WO 322161-2568 threonine. The hydrophobic amino acids include alanine, cysteine, isoleucine, leucine, methionine, phenylalanine, proline, tryptophan, tyrosine and valine. Other families of amino acids include (i) serine and threonine, which are the aliphatic-hydroxy family; (ii) asparagine and glutamine, which are the amide containing family; (iii) alanine, valine, leucine and isoleucine, which are the aliphatic family; and (iv) phenylalanine, tryptophan, and tyrosine, which are the aromatic family. [000171] The term “agent” is used herein to denote a chemical compound, a mixture of chemical compounds, a biological macromolecule, or an extract made from biological materials. [000172] The disclosure also includes F
v, F
ab, F
ab’ and F
(ab')2 anti-CD3 antibody fragments, single chain anti-CD3 antibodies, bispecific anti-CD3 antibodies, heteroconjugate anti-CD3 antibodies, trispecific antibodies, immunoconjugates and fragments thereof. [000173] Bispecific antibodies are antibodies that have binding specificities for at least two different antigens. In the present case, one of the binding specificities is for CD3. The second binding target is any other antigen, and advantageously is a cell-surface protein or receptor or receptor subunit. [000174] All publications and patent documents cited herein are incorporated herein by reference as if each such publication or document was specifically and individually indicated to be incorporated herein by reference. Citation of publications and patent documents is not intended as an admission that any is pertinent prior art, nor does it constitute any admission as to the contents or date of the same. The disclosure having now been described by way of written description, those of skill in the art will recognize that the disclosure can be practiced in a variety of embodiments and that the foregoing description and examples below are for purposes of illustration and not limitation of the claims that follow. EXAMPLES [000175] The following examples are provided for illustration and are not intended to limit the disclosure. Example 1: Effects of Nasal anti-CD3 Antibody on Metabolism [000176] The impact of nasal anti-CD3 on whole-body metabolism in diet induced obese mice was assessed as shown in FIG.1. Male mice (18 weeks old) were fed with a high-fat diet (HFD) ad libidum. Nasal anti-CD3 (1µg/mouse) was administered 3 times per week (Mon, Wed,
Attorney Docket No. TIZI-035/001WO 322161-2568 Fri) for 6 weeks . Controls: PBS (Placebo), or Isotype Control (ISO). Comprehensive Lab Animal Monitoring System (CLAMS) Continuous, real-time measurements were taken. Results Nasal anti-CD3 antibody ameliorates whole body and systemic metabolism without weight loss [000177] Treatment with the nasal anti-CD3 antibody did not affect body weight (FIG.2A) or appetite (FIG.2B). Treatment with the nasal anti-CD3 antibody ameliorated locomotor activity (FIG.3A-3C). Treatment with the nasal anti-CD3 antibody did not affect energy expenditure (FIG.4). Treatment with nasal anti-CD3 antibody increased Respiratory Exchange Ratio (RER), reflecting a metabolic shift towards glucose utilization driven by immune modulation of metabolic inflammation (FIG.5). Glucose and insulin tolerance tests (GTT, ITT) confirmed improved glucose tolerance, insulin sensitivity, and reduced systemic insulin levels, indicating normalized glucose metabolism (FIG.6A-6C). Additionally, serum triglycerides (TG) and cholesterol were reduced, highlighting improved lipid handling (FIG.7). Enhanced liver, kidney, and pancreatic function was evident from reduced levels of Alanine Aminotransferase (ALT), Blood Urea Nitrogen (BUN), Amylase, and Lipase (FIG.8). These results demonstrate that nasal anti-CD3 improves systemic homeostasis in DIO, supporting its potential for treating obesity-associated metabolic inflammation. Example 2: Nasal Anti-CD3 Enhances The Efficacy Of Semaglutide In Ameliorating Diet- induced Obesity Material and methods Mice, diets, and treatments. [000178] C57BL/6J males (5-6 weeks old) were from Jackson laboratory and housed in a controlled environment (12h light/dark cycle, lights on at 3 a.m, 21 ± 2˚C, humidity 50 ± 10%). Each animal was identified by an ear punch. Mice had access to autoclaved tap water and chow diet (3.22 Kcal/g), or 60 Kcal-% fat (D12492, Research diet) for the Diet-induced obesity (DIO) cohort for 18 weeks prior to the start of treatments and for the duration of the treatment period, or Gubra Amylin NASH diet ([GAN diet, 4.49 Kcal/g, 40 kcal-% fat] of these 46% saturated fatty acids by weight, 22% fructose, 10% sucrose, 2% cholesterol; D09100310, Research diet)
Attorney Docket No. TIZI-035/001WO 322161-2568 for the MASH cohort for 34 weeks prior to the start of treatments and for the duration of the treatment period. Mice were randomized for different treatment groups. [000179] Mice were administered with nasal PBS vehicle or anti-CD3 (clone C2-11, 1 µg) three times per week for the indicated periods. Semaglutide (Ozempic™, Novo Nordisk) at low- dose (4.1 µg/Kg/day), or high-dose (41.1 µg/Kg/day) or its NaCl (0.09%, Sigma Millipore) vehicle were subcutaneously injected daily at the period of 4 weeks. Adipose and liver histology. [000180] Perigonadal visceral adipose tissue (VAT), brown adipose tissue (BAT), and liver tissue were fixed overnight in 4% paraformaldehyde, then paraffin or OCT cryo-embedded and sectioned (5um thickness). Liver FFPE sections were stained with hematoxylin and eosin (HE), Picro-Sirius Red (PSR; Sigma-Millipore), alpha smooth muscle actin (α-SMA, cat ab124964, Abcam). Liver cryosections were stained with O-Red Oil (Sigma-Millipore). VAT and BAT sections were stained with F4/80, and Ucp-1, respectively. Serum biochemistry and inflammatory profiling [000181] Total blood was collected from the portal vein of CO2-euthanized mice and kept at +4˚C for 6 hours for serum separation, then serum collected upon a centrifugation for 15min at 3000rpm, aliquoted and stored at -80˚C. Serum samples were analyzed by IDEXX for aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), cholesterol, Lactate dehydrogenase (LDH), and triglycerides (TG), and by mesoscale discovery (Mesoscale Discovery) for the inflammatory cytokines Tumor necrosis factor alpha (TNF-α), and interleukin-1 beta (IL-1β), and the Keratinocyte-derived chemokine (KC) also known as Cxcl1 and for which the human analog is known as Growth-regulated oncogene alpha (GROα). Bulk RNAseq. [000182] Liver and adipose (VAT) tissues were collected in RNAlater (Thermofisher) and stored in -80˚C. Total RNA was extracted following Zymo protocol (Zymo). Ten nanograms of each RNA sample was plated in a prechilled 96-well twin.tec PCR plate LoBind, full skirted plate(Eppendorf, #0030129512) and shipped to the Broad Institute for Smart-seq2 RNA sequencing. Samples were processed for cDNA generation and Illumina Nextera XT library construction. Sequencing data was generated using 2×38 bp paired end sequencing on the NextSeq500. Transcript-level gene expression analysis of the raw RNA-seq reads was
Attorney Docket No. TIZI-035/001WO 322161-2568 performed. First, quality of the raw RNA-seq reads was assessed using FastQC quality control tool for high throughput sequence data. Reads were concatenated then trimmed using Trimmomatic. The RNA reads were aligned to the mouse mm10 reference genome using HISAT2. HISAT2-generated SAM files were sorted and converted into BAM files using Samtools. The sorted reads were assembled into transcripts using StringTie. Next, StringTie- generated transcript lengths and abundance estimates were converted into count matrices using Tximport. Differential gene expression analysis was performed with false discovery rate (FDR)- adjusted P values using DESeq2 with an adjusted P cutoff value of 0.05. Data visualization was performed in R (version 4.0.3). Heatmaps and clustering were generated using heatmap.2 from the gplots package. For clustering, the z-scores were calculated using the mean expression of biological replicates per disease condition and then subsequently clustered using K-means. Pathway analysis was performed using KEGG and ingenuity pathway analysis (IPA) software (Qiagen). Statistical analysis using Qiagen IPA was carried out with a right-tailed Fisher’s exact test. Real time qRT-PCR [000183] RNA was extracted VAT using Zymo kit and reverse transcribed with High- Capacity cDNA Reverse Transcription (Applied Biosystems) according to the manufacturer’s instructions. The cDNA served as a template for amplification of genes of interest using TaqMan probes for Il-10 and Il-6 (Thermo Fisher Scientific) and gene expression was normalized to Gapdh. Flow cytometry [000184] Livers were mechanically processed by trimming the tissue with scissors and were macerated through a 100 μm cell strainer (Fisher Scientific) in cold Roswell Park Memorial Institute (RPMI) 1640 media (Gibco, #11875119) with 10% heat-inactivated 10% fetal bovine serum (FBS; Gibco,#10438026), and centrifuged at 300 g for 5 min at 4°C. Total liver leukocytes were isolated using Percoll Plus gradient protocol, and stained for the indicated surface and intracellular markers. Dead cells were excluded using the fixable viability dye Zombie Aqua (1:1,000; BioLegend) staining. For intracellular cytokine staining, cells were first stimulated for 3 h with PMA (phorbol 12-myristate 13- acetate; 50 ng/mL; Sigma-Aldrich) and ionomycin (1 μM; Sigma-Aldrich) and a protein-transport inhibitor containing monensin (1 μg/mL GolgiStop; BD Biosciences) before detection by staining with the indicated antibodies.
Attorney Docket No. TIZI-035/001WO 322161-2568 Surface markers were stained for 25 min at 4 °C in Mg
2+ and Ca
2+ free Hank's Balanced Salt Solution (HBSS) with 2% PBS, 0.4% Ethylenediaminetetraacetic acid (EDTA) (0.5 M), and 2.5% HEPES (1M) then were fixed in Cytoperm/Cytofix (eBioscience), permeabilized with Perm/Wash Buffer (eBiosciences). Flow-cytometric acquisition was performed on a Symphony A5 instruments (BD Biosciences) by using DIVA soft-ware (BD Biosciences) and data were analyzed with FlowJo software version 10.1 (TreeStar Inc). Glucose and insulin tolerance tests [000185] All WT and knockout DIO males were weighed regularly. After 12 weeks on HFD, fasting blood glucose (OneTouch Ultra) and insulin concentrations (Crystal Chem ELISA) were measured. For glucose tolerance tests, fasted (16 h) mice were given intraperitoneally (i.p.) 0.75–1 g glucose per kg body weight; ITT mice, were given 0.75 U per kg body weight human regular insulin (Eli Lilly). Magnetic resonance imaging [000186] Adipose MRI. Imaging of adipose tissue by magnetic resonance imaging (MRI) was performed on a 3 Tesla Varian Unity/INOVA small-bore MRI system (Varian Inc., Palo Alto, CA). After anesthesia with isoflurane, T1-weighted images were acquired in the coronal plane using a fast spin-echo pulse sequence with TR/TE = 600/12 ms, an echo train length of 2, and 4 averages. A matrix size of 512 × 128 covered a field of view of 60–90 mm × 30 mm with 15 slices of 1.1–1.5 mm thickness separated by a 0.2–0.5 mm gap depending on the size of the animal. A 3D reconstruction of body-wide sub-cutaneous (SAT), visceral adipose tissues (VAT) and cecum was generated using Slicer software, reflecting its shrinkage characteristic of HFD consumption. Then, the total volume of each mouse was quantified to calculate the proportion of scWAT and VAT to total body volume. [000187] Liver spectroscopy.
1H-MRS semi-quantification of liver lipids were performed on a Bruker BioSpec 70/30USR Avance III 7 T horizontal bore MR scanner (Bruker Biospin MRI GmbH, Ettlingen, Germany). During the experiment, all mice were anesthetized with isoflurane/air at 1 to 2%/L/min oxygen) with respiratory monitoring. MR images were collected using multi-slice proton-density-weighted and T2-weighted rapid acquisition with relaxation enhancement sequence with TR=3000 ms, TE1/TE2=9.2/27.6 ms, slice thickness=1mm, number of slices=12, field of view=3.5 × 3.5 cm
2, matrix size=128 × 128, number of excitation (NEX) = 2. The volume of interest of the subsequent prone spectroscopy was carefully located on
Attorney Docket No. TIZI-035/001WO 322161-2568 homogeneous liver parenchyma to avoid contributions from obvious blood vessels, subcutaneous fat, and air. The single-voxel was performed using a point-resolved spectroscopy sequence without water suppression with the following parameters: voxel volume 4 × 6 × 4 mm
3, TR=10000 ms, TE=16.5 ms, 64 signal averages. All MR spectra were processed with Bruker Topspin package. In the mouse model, the distinguished lipid peak was the Lip13 resonance (originating from the (CH2) protons at 1.3 ppm) which represented the major resonance and accounts for approximately 70% of the total lipid signal for fatty liver (17). For this reason, wit was used to quantify the fat content of our animal model. The fat fraction (FF) was calculated from the MR spectra as the ratio of the Lip13 resonance area (Lip13) relative to the water peak area. [000188] Molecular MRI. Animals were anesthetized with isoflurane (1 to 2%) and placed in a specially designed cradle with body temperature maintained at 37°C. The inhaled isoflurane concentration was adjusted to maintain a respiration rate of 60 ± 5 breaths per minute. The tail vein was cannulated for intravenous delivery of the molecular probe while the animal was positioned in the scanner. Imaging was performed at 4.7 T using a small-bore animal scanner with a custom-built volume coil. Mice were imaged with a molecular probe (Fe³⁺-PyC3A) at a dose of 100 μmol/kg from 30 mM stock solution, as determined by ICP-MS. CLAMS analysis [000189] Wild type C57Bl/6 mice were maintained on a high fat diet before entering the comprehensive laboratory animal monitoring system (CLAMS). The mice were singly housed with free access to water and pre-weighed food and acclimated to the system for 48 hours then nasally administered with PBS vehicle or anti-CD3 antibody. These mice were further monitored for 6 weeks. Body weights were measured weekly. The CLAMS measures O2 consumption, CO2 output and heat at 15-min intervals over 6 weeks by indirect calorimetry (Oxymax System, Columbus Instruments). All measurements were normalized to body weight. The CLAMS data was analyzed using a linear mixed models approach in R statistical software version 4.0.5. Prior to analysis, the distribution and normality of the data were tested using graphical methods. Parameters with normal distribution were analyzed with the “glmmTMB” package, while parameters that were not normally distributed were analyzed with the non-parametric mixed model “nlme” package. The glmmTMB package uses maximum likelihood estimation (MLE) and “nlme uses generalized estimating equations to fit generalized linear mixed models
Attorney Docket No. TIZI-035/001WO 322161-2568 (GLMMs) to data. In all models, the treatment group was considered a fixed effect, while day and mouse ID were combined as random effects. The estimates and significance were calculated against the control group's effect (PBS). The statistical significance of the fixed effects was set at a p-value threshold of 0.05. Statistical Analyses [000190] Error bars represent SEM. The statistical significance of differences between two groups was determined using the Mann-Whitney U test or Student’s t tests where appropriate following determination of Gaussian distribution of the data. Differences among mice groups (>2) were evaluated using one-way or two-way ANOVA followed by post hoc Tukey tests. Values of p < 0.05 were considered significant. Results Nasal anti-CD3 antibody restores adipose tissue homeostasis in diet-induced obesity. [000191] FIG.9 shows the effect of nasally administered anti-CD3 antibody (lower panel) compared to controls (top) on liver damage in DIO mice. After normalization, T1 and deltaCNR may be measured. The mice treated with an anti-CD3 antibody showed improved liver state with an increased T1, and a lower delta CNR corresponding a 4.5 fold decrease in inflammation. These results suggest that administration of nasal anti-CD3 ameliorates inflammation and liver damage without Weight Loss. [000192] FIGs.10A-10F show the effects of nasal anti-CD3 antibody on obesity-related inflammation in adipose tissue. FIGs.10A and 10B illustrate the accumulation of fat in the liver in mice treated with anti-CD3 antibody or isotype. Magnetic Resonance Imaging revealed no changes in the volumes of visceral or subcutaneous adipose tissue (VAT and SAT) volumes (FIG.10A), but histological analysis showed fewer crown-like structures, indicating reduced inflammation, with no changes in adipocyte size (FIG.10B). FIG.10C summarizes the KEGG gene pathways that were upregulated in response to treatment with the anti-CD3 antibody. KEGG pathway analysis of RNA sequencing of total adipose tissue demonstrated enhanced insulin signaling, increased arginine metabolism supporting type-2 immunity, and improved protein processing and phagosome pathways, suggesting restored insulin sensitivity (FIG.10C). Quantitative PCR analysis further revealed a downregulation of the pro-inflammatory cytokine Il-6 and increased expression of the anti-inflammatory cytokine Il-10, supporting an anti-
Attorney Docket No. TIZI-035/001WO 322161-2568 inflammatory and pro-regulatory shift in adipose tissue (FIG.10D). These results indicate that Nasal anti-CD3 antibody effectively reduces adipose tissue inflammation and promotes its metabolic homeostasis in the context of diet-induced obesity. Nasal anti-CD3 antibody promotes liver homeostasis in diet-induced obesity. [000193] FIGs.11A-11D show the effects of nasal anti-CD3 antibody administration on obesity-related liver inflammation and fibrosis. Using spectroscopy MRI, a non-invasive method to estimate liver lipid content, it was shown that nasal anti-CD3-treated DIO mice had significantly lower lipid levels compared to controls (FIG.11A). Histopathological analysis further confirmed reduced steatosis in nasal anti-CD3-treated animals (FIG.11B). FIGs 11C and 11D summarize KEGG pathways that are upregulated and downregulated, respectively, in mice treated with anti-CD3 antibody compared to control. Long-term nasal anti-CD3 antibody sustains tissue homeostasis in obesity even post discontinuation. [000194] DIO mice were treated with nasal anti-CD3 or PBS vehicle for 6 weeks, 12 weeks, 12 months, or 6 months followed by a 6-month break. RNA sequencing of liver tissues revealed that nasal anti-CD3 enhanced glutathione and fatty acid metabolism, peroxisome proliferator-activated receptor (PPAR) signaling, oxidative phosphorylation, glycolysis, and fat digestion pathways, indicating improved lipid and glucose metabolism in the liver (FIG.11C). Conversely, pathways associated with Th17, Th1, and Th2 differentiation, NK cell-mediated cytotoxicity, and pro-inflammatory signaling, including TNFα, were downregulated, suggesting reduced pro-inflammatory and cytotoxic lymphocyte activity in the liver. Furthermore, markers of liver apoptosis and senescence were also downregulated, underscoring the restoration of liver tissue homeostasis (FIG.11D). Thus, our data support that nasal anti-CD3 effectively limits early MAFLD progression by reducing fat accumulation and inflammation while restoring liver homeostasis in diet-induced obesity. [000195] FIG.12 shows differentially expressed genes in cells treated with anti-CD3 antibody compared to isotype control and RNA sequencing of liver tissues revealed that nasal anti-CD3 antibody triggered over 1000 differentially expressed genes that persisted across all time points, including 6 months post-treatment (modules U vs D). However, distinct signatures were more prominent in specific stages of the treatment. Early stages were characterized by upregulated genes (modules U1, U2, U3 and U4), while downregulated genes became dominant in
Attorney Docket No. TIZI-035/001WO 322161-2568 later stages (modules D1, D2, D3, and D4) (FIG.12). Nasal anti-CD3 antibody reduced inflammation (Socs6 and Cxcl16 up, Cxcl12 and Tnfrsf12a down), improved lipid handling (Acadvl and Lpin2 up, Fasn, Acsl1, and Cidec down), normalized glucose metabolism (Foxo1 and Insr up, G6pc1 down), enhanced liver regeneration (Angptl6 and Timp3 up), decreased cell death (Bcl2l12 up, Hif1a down), attenuates fibrosis (Srebf2 up, Col6a1 down) and reduces oxidative stress (Cox6a1 down). GO/IPA pathway analysis further underscored anti-CD3 antibody-mediated reductions in liver toxicity (data not shown). Histopathological analysis confirmed the resolution of steatosis and steatohepatitis during prolonged nasal anti-CD3 antibody treatments. Notably, livers from DIO mice left untreated for 6 months post-treatment displayed better tissue homeostasis than vehicle-treated mice, though less optimized than those treated continuously for 12 months (data not shown). Together, these findings position nasal anti-CD3 antibody as a unique immunotherapy capable of maintaining long-term tissue homeostasis without cytotoxicity, even after treatment discontinuation. Example 3: Effects Of The Combination of Nasal Anti-CD3 With Semaglutide In A Diabetes Induced Obesity Model Materials and Methods [000196] Experiments were conducted as described in Example 2. DIO mice were treated with nasal anti-CD3 antibody or PBS vehicle for 2 weeks, followed by combined treatment with low-dose (LD) or high-dose (HD) semaglutide or NaCl vehicle for 4 weeks. Results Nasal anti-CD3 antibody amplifies low-dose semaglutide-induced homeostasis and outperforms high-dose semaglutide in diet-induced obesity [000197] FIG.13 shows the effect of body weight in mice treated with anti-CD3 antibody only or with anti-CD3 antibody in combination with semaglutide. Body weight monitoring showed no weight loss during the initial 2 weeks of nasal anti-CD3 antibody treatment alone. When combined with semaglutide, nasal anti-CD3 antibody did not interfere with semaglutide- induced weight loss, which was greater with HD compared to LD, as expected. [000198] FIG.14 shows the effects of the combination of anti-CD3 antibody and semaglutide on liver integrity and liver inflammation.
Attorney Docket No. TIZI-035/001WO 322161-2568 [000199] Advanced molecular MRI using an iron-based inflammation and oxidative stress probe (Fe³⁺-PyC3A) measured via ∆CNR revealed that nasal anti-CD3 antibody combined with low-dose (LD) semaglutide reduced liver inflammation by over 22-fold compared to LD alone (∆CNR=0.09 vs ∆CNR=2.08). Additionally, T1 measurement of liver integrity improved (T1=777 vs T1=922), and the fat fraction (FF) was significantly reduced. [000200] FIG.15 shows images of the livers of mice treated with anti-CD3 antibody and/or semaglutide in high doses (HD) or low doses (LD), as well as tissue slices. The graph shows a quantitative histopathological score of liver homeostasis. Histopathological analysis of the livers revealed that neither low-dose (LD) nor high-dose (HD) semaglutide alone improved MAFLD pathology. However, the combination with nasal anti-CD3 antibody significantly ameliorated pathology, with the LD combination outperforming HD semaglutide alone. These data indicate that administration of nasal anti-CD3 antibody enhances semaglutide-mediated liver homeostasis in DIO mice. [000201] FIG.16 shows the effect of the combination of anti-CD3 antibody and semaglutide on lipid accumulation in the liver. Addition of anti-CD3 antibody to high dose semaglutide decreased the lipid accumulation in the liver. Oil Red O analysis of intracellular lipid content suggests that nasal anti-CD3 antibody enhances semaglutide’s efficacy in improving hepatocyte lipid handling. [000202] FIG.17 shows results of a gene expression analysis of genes associated with liver inflammation and remodeling in cells treated with low dose semaglutide and high dose semaglutide alone or in combination with anti-CD3 antibody. Remarkably, RNAseq analysis of these livers, alongside those from age-matched DIO mice treated with nasal anti-CD3 antibody alone versus PBS vehicle (FIG.11C), revealed that nasal anti-CD3 antibody is the primary driver amplifying semaglutide’s efficacy. This effect is achieved through taming liver inflammation (Ccl19, Ccl2 and Cxcl12 down), upregulating lipid oxidation (Cpt1a, Ppargc1b, and Ppargc1a up, Fabp1 down), promoting insulin sensitivity (Insr and Gck up), normalizing glucose metabolism, and reducing cell death (Bcl2l1 up, Bax and Diablo down) (FIG.5F). Consistently, nasal anti-CD3 antibody-mediated enhancement of semaglutide’s effects extended to visceral adipose tissue (VAT). [000203] FIG.18 shows the levels of F4/80+ cells in adipose tissue from mice treated with high dose (HD) or low dose (LD) semaglutide alone and/or anti-CD3 antibody. F4/80+ cells are
Attorney Docket No. TIZI-035/001WO 322161-2568 macrophages, indicating inflammation. Crown-like structures (CLS), a hallmark of adipose inflammation, were significantly reduced when nasal anti-CD3 antibody was combined with semaglutide. [000204] FIG.19 shows results of a gene expression analysis of genes associated with metabolic pathways in liver samples from mice treated with low dose semaglutide and high dose semaglutide, respectively, alone or in combination with anti-CD3 antibody. Expression of genes associated with inflammation and lipid metabolism tended to decrease with semaglutide treatment compared to control and further when semaglutide was combined with anti-CD3 antibody. These data show that nasal anti-CD3 enhances semaglutide-mediated adipose homeostasis in DIO mice. [000205] RNAseq analysis of VAT quantitatively captured that the combination of nasal anti-CD3 antibody with low-dose (LD) semaglutide outperformed high-dose (HD) semaglutide in reducing inflammation (Ccl2 and Cxcl12 down), improving lipid (Pparg and Ppargc1b up) and glucose (Gpx1 and Irs1 up, Xbp1 down) metabolism, promoting tissue regeneration (Ecm2 and Egfr up), and limiting cell death (Casp3 and Casp9 down) (FIG.19). [000206] FIG.20 shows the levels of UCP-1 in adipose tissue from mice treated with high dose (HD) or low dose (LD) semaglutide alone and/or anti-CD3 antibody. UCP-1 is a maker of thermogenic adipocytes. Nasal anti-CD3 antibody-driven tissue homeostasis extends to other tissues by upregulating Ucp-1, enhancing semaglutide-induced thermogenesis in brown adipose tissue and further supporting its insulin-sensitizing effects. [000207] FIGs.21A-21C show the levels of TNFα, IL-1β and KC/GRO, respectively, in serum samples from mice treated with treated with high dose (HD) or low dose (LD) semaglutide alone and/or anti-CD3 antibody. These markers measure systemic inflammation. FIGs.22A-22E show expression levels of AST, BUN, cholesterol, LDH, and triglycerides, respectively, in serum samples from mice treated with treated with high dose (HD) or low dose (LD) semaglutide alone and/or anti-CD3 antibody. These markers measure systemic metabolism, and all markers decreased with semaglutide treatment. At the systemic level, the combination of nasal anti-CD3 antibody with low dose (LD) semaglutide normalized metabolic inflammation, with TNFα levels (FIGs.21A-21C), as well as AST, BUN, cholesterol, LDH, and TG levels, comparable to those of chow-fed healthy control mice (FIGS.22A-22E). These findings highlight nasal anti-CD3 antibody as a primary driver amplifying the efficacy of low-dose
Attorney Docket No. TIZI-035/001WO 322161-2568 semaglutide, surpassing high-dose semaglutide in improving tissue and systemic homeostasis in diet-induced obesity. Example 4: Effects Of The Combination Of Nasal Anti-CD3 With Semaglutide In a Model Of Fibrosis Materials and Methods [000208] Experiments were conducted as described in Example 2. Results Nasal anti-CD3 antibody reverses MASH-related liver fibrosis independently of semaglutide [000209] FIG.23 shows the levels of fibrosis as determined by Sirius Red stain in liver samples from mice treated with treated with semaglutide (HD) and/or anti-CD3 antibody. [000210] FIG.24 shows the levels of stellate cells in liver tissue from mice treated with semaglutide (HD) and/or anti-CD3 antibody. Stellate cells have a key role in the initiation, progression, and regression of liver fibrosis. Sirius Red staining and histopathological scoring revealed that nasal anti-CD3 antibody alone significantly ameliorated MASH-related fibrosis, whereas semaglutide alone had no effect. Notably, adding nasal anti-CD3 antibody to semaglutide improved semaglutide's efficacy, but the combination achieved outcomes comparable to nasal anti-CD3 antibody alone, underscoring nasal anti-CD3 antibody as the primary driver of fibrosis reversal in advanced MAFLD (FIG.23). Similarly, smooth muscle actin alpha (SMA-α) staining, a hallmark of stellate cell activation and a key mediator of liver fibrosis, revealed that nasal anti-CD3 antibody alone substantially suppressed stellate cell activation. This effect was maintained when combined with semaglutide, whereas semaglutide alone had a significantly weaker effect (FIG.24). [000211] RNAseq analysis of livers from MASH mice revealed that nasal anti-CD3 antibody alone drives the resolution of inflammation (Ccl9 and Il6ra down), normalization of lipid (Cpt1a and Lpin2 up) and glucose (Prkag1 and G6pc1 up) metabolism, and reduction of cell death (Casp3 and Bax down) and fibrosis (Mmp14 and Col3a1 down). alone had minimal effects on these pathways, but its combination with nasal anti-CD3 antibody conferred these abilities, underscoring the added value of nasal anti-CD3 antibody in mitigating MASH-related
Attorney Docket No. TIZI-035/001WO 322161-2568 liver injuries (FIG.25A). Similar results were observed in RNAseq analysis of VAT from MASH mice (FIG.25B). [000212] FIG.26 shows levels of CD45+/IL10+ liver cells in mice treated with treated with semaglutide and/or anti-CD3 antibody. IL-10 is immunoregulatory and prevents stellate activation, while promoting tissue repair. Levels of CD45+/IL10+ cells were increased by treatment with semaglutide and further increased after treatment with semaglutide in combination with anti-CD3 antibody compared to semaglutide treatment alone. These data suggest synergistic immunoregulatory and anti-stellate activation effects of nasal anti-CD3 and semaglutide in MASH mice. [000213] FIGs.27A and 27B shows result of an Ingenuity Pathway Analysis (IPA) of liver function. The Venn diagram shows the number of differentially expressed genes in total liver tissue between mice treated with anti-CD3 antibody and controls, between mice treated with anti-CD3 antibody and semaglutide and between mice treated with a combination of anti-CD3 antibody and semaglutide compared to mice treated with semaglutide alone. These data suggest that nasal anti-CD3 antibody promotes liver regeneration and downregulates liver cell death independently of semaglutide. [000214] FIGs.28A-28C show levels of lipid metabolism and liver function markers ALT, cholesterol and triglycerides, respectively, in the blood of mice treated with semaglutide and/or anti-CD3 antibody. FIGs.29A-29C show blood levels of inflammatory markers TNFα, IL-1β and KC/GRO, respectively, in the blood of mice treated with semaglutide and/or anti-CD3 antibody. At the systemic level, nasal anti-CD3 antibody alone demonstrated comparable efficacy in normalizing metabolism (FIGs.28A-28C) and metabolism (FIGs.29A-29C). Notably, this effect was maintained when nasal anti-CD3 antibody was combined with semaglutide. Together, our data support nasal anti-CD3 antibody as a primary driver in reversing MASH-related liver fibrosis and restoring systemic and tissue-specific homeostasis in advanced MAFLD, with semaglutide benefiting from its combination with nasal anti-CD3 antibody. Nasal anti-CD3 antibody-driven T cell modulation reshapes myeloid compartments to support tissue homeostasis in obesity-related complications. [000215] Since nasal anti-CD3 antibody restores adipose and liver homeostasis across early and advanced stages of obesity-related complications, it was hypothesized that its targeted modulation of the T cell compartment rehabilitates tissue-remodeling macrophage polarization to
Attorney Docket No. TIZI-035/001WO 322161-2568 sustain tissue homeostasis. Flow cytometry analysis of visceral adipose tissue (VAT) and liver from DIO mice treated with nasal anti-CD3 antibody or isotype control for 6 weeks showed an expansion of Tregs in VAT and spleen (FIG.30A), and a reduction in the frequency of CD8 and Th17 cells in the liver (FIG.30B), which associated with an enrichment in tissue-remodeling (M2) macrophages in VAT (FIG.30C) and a decrease of proinflammatory monocyte-derived macrophage and neutrophils in the liver (FIGs.30D and 30E). In MASH mice, it was found that nasal anti-CD3 antibody, but not semaglutide, expanded tissue remodeling Tim4+ Kupffer cells and reduced pro-inflammatory Tim4- monocytes-derived macrophages (FIG.30F). Leukocytes isolated from the livers of MASH mice showed increased IL-10 production in response to semaglutide or nasal anti-CD3 antibody alone, with further upregulation observed when both treatments were combined. These findings demonstrate that nasal anti-CD3 antibody restores adipose and liver tissue homeostasis across early and late stages of obesity-related inflammation through targeted T cell modulation, promoting tissue-remodeling macrophages, with IL-10 potentially playing a contributing role. [000216] FIGs.31A and 31B show decreases in inflammatory cells in livers of mice treated with anti-CD3 antibody compared to controls. FIGs.31C and 31D show levels of ALT and BUN after prophylactic and therapeutics treatment with the nasal anti-CD3 antibody. The anti-CD3 antibody treatment has both a prophylactic as well as a therapeutic effect on CCL4-induced fibrosis, ALT and BUN. These results indicate that nasal anti-CD3 antibody ameliorates obesity- related liver inflammation and reverses fibrosis. Discussion of Examples 1-4 [000217] Obesity and its associated disorders, including type-2 diabetes, metabolic- associated fatty liver disease (MAFLD) and the more advanced metabolic-associated steatohepatitis (MASH), arise in part from chronic, low-grade inflammation within key metabolic tissues such as the liver and adipose depots. In this study, both early and advanced diet-induced obesity preclinical mouse models were employed to examine the therapeutic potential of nasal anti-CD3 (anti-CD3 antibody) immunotherapy. The findings reveal that anti- CD3 antibody reshapes the immuno-metabolic environment, restoring tissue homeostasis, reducing insulin resistance, and improving systemic metabolic parameters. Notably, anti-CD3 antibody not only prevents early-stage MAFLD progression but also attenuates established MASH-related fibrosis, thereby shifting the tissue milieu toward a more regenerative and less
Attorney Docket No. TIZI-035/001WO 322161-2568 inflammatory state. Importantly, these benefits extend beyond the liver, enhancing adipose tissue integrity and, when combined with sub-therapeutic (low-dose) semaglutide, yielding metabolic improvements that exceed those achieved by high-dose semaglutide alone. [000218] A key observation from the work presented in Examples 1-4 is that nasal anti- CD3 antibody confers these metabolic and tissue-level benefits independently of weight loss. While weight reduction remains a clinical cornerstone for ameliorating metabolic dysfunction, our data indicate that targeted immunomodulation alone can normalize glycemia, enhance insulin sensitivity, improve lipid profiles, and mitigate inflammation and fibrosis without significant alterations in body weight. This dissociation underscores the potential of immunotherapeutic strategies to address persistent metabolic dysfunction that is not solely dependent on changes in adiposity and BMI. [000219] Mechanistically, given the specificity of anti-CD3 monoclonal antibody in targeting T cells, the findings point toward a T cell-driven remodeling of the myeloid compartment as central to the observed improvements. By increasing regulatory T cell (Treg) abundance and diminishing pro-inflammatory T cell subsets, such as Th17 and activated CD8+ T cells, anti-CD3 antibody creates an immunological milieu that favors tissue-remodeling (M2- like) macrophage phenotypes. This cellular realignment contributes to maintaining tissue integrity, enhancing insulin sensitivity, and limiting inflammation-driven injury. Crucially, these immunological and metabolic benefits are seen at both early and advanced stages of obesity- related liver disease, demonstrating the versatility of anti-CD3 antibody’s therapeutic potential. [000220] The synergy of anti-CD3 antibody with semaglutide, a glucagon-like peptide-1 receptor agonist (GLP-1RA), further highlights the promise of immunomodulatory interventions. Pairing anti-CD3 antibody with a low dose of semaglutide produced superior metabolic outcomes to those achieved by high dose semaglutide alone. Without wishing to be bound by theory, it is hypothesized that by alleviating the load on the GLP-1 axis, anti-CD3 antibody- driven immune axis, enables robust metabolic improvements at lower drug dosages, potentially minimizing dose-related side effects and broadening the therapeutic window of GLP-1RAs and related polygenists. Remarkably, these immunometabolic benefits persisted beyond the treatment period, suggesting that anti-CD3 antibody confers durable metabolic reprogramming within affected tissues and may help prevent the rebound inflammation and metabolic decline often observed after cessation of GLP-1RAs or other interventions.
Attorney Docket No. TIZI-035/001WO 322161-2568 [000221] Data presented in Examples 1-4 also emphasize the long-lasting effects of anti- CD3 antibody even post-discontinuation, as prolonged administration-maintained tissue homeostasis and attenuated inflammation and cellular injury. Given that obesity and its metabolic sequelae are characterized by entrenched inflammatory memory, the ability of anti- CD3 antibody to sustain improvements beyond active therapy is clinically significant. For instance, patients may achieve substantial weight reduction through bariatric surgery or pharmacotherapy yet continue to harbor residual inflammation, insulin resistance, and fibrotic remodeling. By mitigating these persistent immunoinflammatory processes, anti-CD3 antibody could play an essential role as an adjunct therapy in preventing the progression or reemergence of metabolic complications, including those associated with advanced MASH, even in individuals who have achieved a normal weight status. [000222] From a clinical perspective, these findings open multiple avenues. First, anti-CD3 antibody-based immunotherapy addresses a key unmet need: correcting metabolic dysregulation in patients who cannot achieve or maintain substantial weight loss. Second, by enhancing the potency of GLP-1RAs at reduced doses, anti-CD3 antibody may lower the burden of pharmacotherapy-associated side effects and improve patient adherence. Third, the sustained benefits observed after therapy discontinuation raise the possibility of finite treatment courses that still confer durable metabolic stabilization—an attractive proposition for managing chronic metabolic diseases. [000223] Nasal anti-CD3 antibody immunotherapy is a promising next-generation strategy to ameliorate obesity-associated comorbidities, both as a standalone intervention and in synergy with emerging GLP-1RAs. Other Embodiments [000224] While the disclosure has been described in conjunction with the detailed description thereof, the foregoing description is intended to illustrate and not limit the scope of the disclosure, which is defined by the scope of the appended claims. Other aspects, advantages, and modifications are within the scope of the following claims.