86249859 1 ULTRAVIOLET LIGHT ACTIVE PARTICLE FOR TONER COMPOSITION BACKGROUND [0001] Distinguishing a genuine toner product from a counterfeit toner product is challenging for several reasons. The use of sophisticated packaging and imitation security features (e.g., holograms, serial numbers and/or tamper evident seals) can lead to the impression of a genuine toner product. Empty toner cartridges of genuine toner products can also be “refilled” and then “repackaged” for sale, once again giving the impression that a genuine toner product is being purchased. [0002] To help identify a genuine toner product, manufacturers have attempted to use a variety of toner additives or features. For example, magnetic particles or additives, microscopic taggants and/or markers have all been used in toner formulations, but these require specialized equipment or detection methods that are inconvenient for quickly authenticating the toner in question. Luminescent agents have also been used, but among other things the addition of luminescent agents to toner formulations can negatively impact the print quality of the toner product along with providing less than effective luminescence due to the agglomeration of the luminescent agents (e.g., quenching of the desired luminescent response). DETAILED DESCRIPTION [0003] An ultraviolet light active particle for use with a toner particle to provide a toner composition is described herein. The ultraviolet light active particles are disposed on the exterior surface of the toner particle to provide the toner composition. Each of the ultraviolet light active particles has a spherical core/shell structure. The core contains an ultraviolet light active compound that emits light in the visible spectrum when exposed to ultraviolet light. The shell is substantially free of the ultraviolet light active compound and serves to bind the particle to the exterior surface of the toner particle. [0004] The shell also serves to space each of the ultraviolet light active particles on the surface of the toner particle to minimize or eliminate quenching
86249859 2 of the luminescent response while also improving the quantum yield of the luminescent response. This is an advantage of the present disclosure in that the spacing of the ultraviolet light active particles on the surface of the toner particle provided by the shell results in a quantum yield that is higher than if the ultraviolet light active compounds were simply added to the toner particles. When luminescent particles are in too close a proximity to each other (e.g., if the ultraviolet light active compound where simply added to the toner particles), there is an increased likelihood of quenching due to energy transfer processes or other interactions between neighboring particles. These interactions can lead to non- radiative energy dissipation, reducing the overall luminescent response. [0005] In contrast, the shell of the ultraviolet light active particle of the present disclosure helps to space each core (containing the ultraviolet light active compound) from each other on the surface of the toner particle such that the light emission in the visible spectrum is not significantly impacted by surrounding ultraviolet light active particles. In other words, by increasing the physical spacing between the core containing the ultraviolet light active compound via the shell according to the present disclosure the quenching interactions is reduced. This affect can be supported by the data in Table 1 of the Examples section below, where the relative intensity of emission for the Examples is seen to be significantly higher relative to the Comparative Examples having the same weight percentage of the ultraviolet light active compound. This higher relative intensity of emission corresponds to a higher quantum yield in that a brighter luminescent signal is the result of more photons being emitted per unit of absorbed energy. [0006] The ultraviolet light active particles on the surface of the toner particle also provide the resultant toner composition with enhanced performance both in terms of print quality and ease of verifying authenticity (e.g., use of readily available and convenient ultraviolet light source) as compared to other approaches such as those that employ luminescent agents in the toner formulations. [0007] The ultraviolet light active particle herein includes the core formed of a first polymer and an ultraviolet light active compound and the shell formed of a second polymer at least partially surrounding the core. In various examples,
86249859 3 the shell is substantially free of the ultraviolet light active compound, and the core of the ultraviolet light active particle absorbs ultraviolet light and emits visible light. The term “substantially free” when used in this context, means that the shell contains less than 2 % by weight (wt.%), or less than 1 wt.%, or less than 0.5 wt.% or 0 wt.% of the ultraviolet light active compound based on the weight of the shell. When the shell is “free” of the ultraviolet light active compound, this means the shell contains 0 wt.% of the ultraviolet light active compound based on the weight of the shell. [0008] The core of the ultraviolet light active particle can absorb ultraviolet light at wavelengths, for example, 200 nm to 350 nm (short and medium wave ultraviolet) and expresses a specific visible light at, for example, a wavelength of 400 nm to 550 nm (blue and green). This difference in color can vary with the presence or absence of ultraviolet light, making it easy and accurate to confirm the authenticity of the toner composition. This confirmation process can be checked in the solid state and the printed state without an additional discrimination method. In addition, the ultraviolet light active particles are added to the toner particle, such that the toner composition with ultraviolet light active particles may be provided without changing toner manufacturing processes. [0009] The core of the ultraviolet light active particle can be formed using a variety of techniques. For example, the core can be formed using emulsion polymerization. Emulsion polymerization is a technique for producing polymer particles with controlled sizes. Generally, the emulsion polymerization process involves the use of at least one monomer for forming the first polymer of the core along with an emulsifier to help stabilize droplets of the monomer in a continuous aqueous phase (e.g., water). An initiator can also be used to initiate the polymerization of the first polymer, where the initiator generates free radicals that initiate the polymerization process. [0010] Monomers used in forming the first polymer of the core can be selected from the group consisting of styrene, methyl methacrylate (MMA) and combinations thereof. Other monomers are also possible to use in combination with styrene and/or MMA, where examples include ethyl acrylate, allyl methacrylate, ethyl methacrylate (EMA), butyl methacrylate (BMA), isobutyl
86249859 4 methacrylate (IBMA), glycidyl methacrylate (GMA), hydroxyethyl methacrylate (HEMA), vinyl methacrylate (VMA), alpha-methylstyrene (AMS), vinyltoluene, and p-methylstyrene. The monomer concentration in the emulsion polymerization can range from 10 wt.% to 50 wt.% (w/w) relative to the total weight of the emulsion mixture, where lower monomer concentration values can help to achieve the smaller particle sizes discussed herein. [0011] The core further includes the ultraviolet light active compound. The ultraviolet light active compound is selected from the group consisting of benzoisoquinoline, benzothiazole, benzoxazole, 6-methoxy-2-(octan-3-yl)-1H- benzo[de]isoquinoline-1,3(2H)-dione, and combinations thereof. The ultraviolet light active compound concentration in the emulsion polymerization can range from 0.01 wt.% to 0.1 wt.% (w/w) relative to the total weight of the emulsion mixture. All Individual values and sub-ranges from 0.01 wt.% to 0.1 wt.% are included. For instance, in some examples the ultraviolet light active compound concentration in the emulsion polymerization can range from a lower value equal to 0.01, 0.025, or 0.05 to an upper value equal to 0.2, 0.5 or 1.0 weight percent based on the total weight of the total weight of the emulsion mixture, among other possibilities. [0012] Emulsifiers can be selected from those that stabilize the monomer droplets in the continuous aqueous phase (e.g., water). Examples of emulsifiers include sodium dioctyl sulforsuccinate (SDOSS), dodecylmercaptane, sodium dodecyl sulfate (SDS) or polyvinyl alcohol (PVA). Examples of other possible emulsifiers include cetyltrimethylammonium bromide (CTAB), Triton™ X-100 (DOW® Inc.) and sorbitan esters, among others. The amount of emulsifier used in the emulsification reaction can vary depending on factors such as the specific emulsifier, the monomer(s) being emulsified and the desired stability of the emulsion. The amount of the emulsifier used can range from 0.1 wt.% to 5 wt.% (w/w) based on the total weight of the emulsion formulation. [0013] In forming the emulsion, the monomer(s) for forming the first polymer, as provided herein, and the ultraviolet light active compound are mixed with the continuous aqueous phase (e.g., water) and the emulsifier in a reaction vessel. Examples of such reaction vessels include a stirred tank reactor (STR)
86249859 5 (e.g., a batch STR or a continuous STR) equipped with agitation, plug flow reactors (e.g., a tubular reactor), a semi-batch reactor, or a multistage reactor. The level of agitation to achieve the desired particle size is sufficiently high to achieve efficient mixing and uniform dispersion of the monomer phase within the continuous aqueous phase while avoiding excessive shear that results in droplet coalescence or emulsifier degradation. Agitation can be provided by various devices, such as mechanical stirring, high-speed homogenizers, impellers, or other mixing devices. The emulsion is typically agitated for a sufficient duration to achieve adequate monomer dispersion and emulsion stability before initiating the polymerization reaction. The duration of agitation may range from several minutes to several hours. [0014] The initiator helps to initiate the polymerization to form the first polymer, where suitable initiators include potassium persulfate (KPS), azobisisobutyronitrile (AIBN) and/or ammonium persulfate (APS). Typical initiator concentrations include 0.1 wt.% to 5 wt.% (w/w) based on the total weight of the monomer phase in the emulsion. [0015] Polymerization temperatures for the emulsion polymerization of the core can range from 60 °C to 80 °C. Polymerization times in forming the core can range from several minutes to several hours, where the reaction time is sufficient to allow for the desired particle growth and stabilization. Upon completion of the polymerization time the emulsion is cooled to below the reaction temperature to stop the polymerization. The cores are collected through any number of techniques, including filtration, centrifugation, or evaporation to separate them from the remaining aqueous phase. The core can then be washed to remove residual impurities and/or unreacted monomers and then dried to obtain a powder or solid form. [0016] The core can provide 55 wt.% to 95 wt.% of the ultraviolet light active particle based on the total weight of the ultraviolet light active particle. All individual values and sub-ranges from 55 wt.% to 95 wt.% are included. For instance, in some examples the core can range from a lower value equal to 55, 65 or 75 wt.% to an upper value equal to 80, 85.90 or 95 wt.% based on the total weight of the ultraviolet light active particle, among other possibilities.
86249859 6 [0017] The emulsion polymerization method(s) described herein are used to form the shell around the core. For example, the shell can be formed around the core using seeded emulsion polymerization, where the core is used as the seed for the growth of the shell. The cores, as described herein, of the desired size, achieved through filtration or centrifugation, are washed to remove impurities. The monomer for forming the second polymer of the shell is then selected from the monomers provided herein for the first polymer. The monomer concentration in the emulsion polymerization for forming the second polymer can range from 10 wt.% to 50 wt.% (w/w) relative to the total weight of the emulsion mixture. [0018] The monomer selected for the second polymer of the shell can be the same or different than the one(s) used in forming the first polymer of the core. For example, the first polymer and the second polymer are formed from monomers selected from the group consisting of styrene, methyl methacrylate (MMA) and combinations thereof. As discussed herein, other monomers are also possible to use in combination with styrene and/or MMA, where examples include ethyl acrylate, allyl methacrylate, ethyl methacrylate (EMA), butyl methacrylate (BMA), isobutyl methacrylate (IBMA), glycidyl methacrylate (GMA), hydroxyethyl methacrylate (HEMA), vinyl methacrylate (VMA), Į-methylstyrene (AMS), vinyltoluene, and p-methylstyrene. In one embodiment, identical monomers are used to form both the first polymer and the second polymer. For example, both the first polymer and the second polymer can be formed from a master batch of monomer(s). So, both the first polymer and the second polymer can be formed of a master batch of styrene monomers. Alternatively, the first polymer and the second polymer can be formed from a master batch of MMA monomers. [0019] To prepare the shell, the monomer(s) for forming the second polymer is emulsified in a matter similar to the emulsion forming the first polymer of the core. The second polymer is prepared by mixing the monomer(s) for forming the second polymer, as provided herein, in a continuous aqueous phase (e.g., water) and the emulsifier, as provided herein, in a reaction vessel. Examples of such reaction vessels with agitation are provided herein. The emulsion is typically agitated for a sufficient duration to achieve adequate
86249859 7 monomer dispersion and emulsion stability before initiating the polymerization reaction. The duration of agitation may range from several minutes to several hours. [0020] The cores are then introduced into the emulsion of the monomers for forming the second polymer of the shell, where the core acts as the nucleation site for the growth of the second polymer of the shell. The initiator, as provided herein, is then added to the emulsion, where the type and amount of initiator for forming the second polymer of the shell are as described for the first polymer of the core. The polymerization temperatures for the emulsion polymerization of the second polymer for the shell can range from 60 °C to 80 °C. Polymerization times in forming the shell can range from several minutes to several hours, where the reaction time is sufficient to allow for the desired shell growth and stabilization. The shell formed of the second polymer at least partially surrounding the core. The shell formed of the second polymer can also completely surround the core. As discussed herein, the shell does not contain (e.g., is free of) the ultraviolet light active compound. [0021] The shell can provide 5 wt.% to 45 wt.% of the ultraviolet light active compound based on the total weight of the ultraviolet light active particle. All individual values and sub-ranges from 5 wt.% to 45 wt.% are included. For instance, in some examples the shell can range from a lower value equal to 5, 10, 20 or 30 wt.% to an upper value equal to 40, 42.5 or 45 wt.% based on the total weight of the ultraviolet light active particle, among other possibilities. [0022] In accordance with various examples, the emulsion is cooled to below the reaction temperature to stop the polymerization upon completion of the polymerization time. The resulting ultraviolet light active particles may be collected through any number of techniques, including filtration, centrifugation, salting out, or evaporation to separate them from the remaining aqueous phase. In the salting out process, once the emulsion polymerization is complete and cooled to below the reaction temperature (e.g., room temperature) salt is gradually added to the cooled reaction mixture with stirring for a sufficient duration to allow the ultraviolet light active particles to aggregate and settle. Examples of suitable salts include sodium chloride (NaCl) or calcium chloride (CaCl2). After
86249859 8 settling, the supernatant liquid is decanted and the ultraviolet light active particles are washed to remove residual impurities and/or unreacted monomers and then dried to obtain a powder or solid form. [0023] The ultraviolet light active particles can have an average particle diameter in a range from about 5 nanometers (nm) to about 1 micrometer (μm). All individual values from 5 nm to 1 μm are included. For instance, the ultraviolet light active particles can have an average particle diameter of about 10 nm to about 1 μm, or of about 50 nm to about 500 nm, among other possible values. As used herein, the average particle diameter refers to or includes the diameter of a spherical particle, or the average diameter of a non-spherical particle (e.g., the average of multiple diameters across the non-spherical particle). The average particle diameter of particles may be measured by a particle diameter distribution measuring device (manufacturer: MICROTRAC, trade name: NANOTRAC FLEX). The average particle diameter of the ultraviolet light active particles may be: (a) calculated as an average of a plurality of the ultraviolet light active particles (e.g., all the particles or a sample of the particles) or (b) calculated as the average diameter of an individual particle (e.g., the average of multiple diameters across a non-spherical particle). [0024] The ultraviolet light active particles can also have an average particle sphericity of about 0.93 to 1.0, where sphericity is a measure of how closely an object (e.g., the ultraviolet light active particle) resembles a sphere in terms of its roundness or circularity, where a value of 1 represents a perfect sphere while values closer to 0 indicate shapes that deviate significantly from a sphere and are more elongated, irregular, or angular. The sphericity of the ultraviolet light active particles can be measured by a flow particle image analyzer, FlowCam® 8000 manufactured by YOKOGAWA Corp. using an analysis software (VisualSpreadsheet® such as version 5.9.1.78). [0025] The toner composition provided herein may include a toner particle having an exterior surface and one or more of the ultraviolet light active particle disposed on the exterior surface of the toner particle. The toner composition absorbs ultraviolet light and emits visible light, as discussed herein. The toner particle may include a binder resin, a releasing agent, and a pigment. According
86249859 9 to one aspect of the present disclosure, the toner composition can be used for developing an electrostatic image. [0026] Examples of the binder resin may include, but are not limited to, a styrenic resin, an acrylic resin, a vinyl resin or polyolefin resin, a polyether-based polyol resin, a phenolic resin, a silicone resin, a polyester resin, an epoxy resin, a polyimide resin, a polyurethane resin, a polybutadiene resin, or any mixture thereof. [0027] Examples of the styrenic resin may include, but are not limited to, polystyrene, a homopolymer of a styrenic monomer such as poly-p-chlorostyrene or polyvinyltoluene, a styrene-based copolymer such as a styrene-p- chlorostyrene copolymer, a styrenevinyltoluene copolymer, a styrene-vinyl naphthalene copolymer, a styrene-acrylic acid ester copolymer, a styrene- methacrylic acid ester copolymer, a styrene-methyl achloromethacrylate copolymer, a styrene-acrylonitrile copolymer, a styrene-vinyl methyl ether copolymer, a styrene-vinyl ethyl ether copolymer, a styrene-vinyl methyl ketone copolymer, a styrene-butadiene copolymer, a styrene-isoprene copolymer, or a styrene-acrylonitrile-indene copolymer, or any mixture thereof. [0028] Examples of the acrylic resin may include, but are not limited to, a polymer of acrylic acid, a polymer of methacrylic acid, a polymer of methyl methacrylate, a polymer of methyl Į-chloromethacrylate, or any mixture thereof. [0029] Examples of the vinyl resin or polyolefin resin may include, but are not limited to, polyvinyl chloride, polyethylene, polypropylene, polyacrylonitrile, polyvinyl acetate, or any mixture thereof. [0030] The polyester resin may be prepared via reaction between an aliphatic, alicyclic, or aromatic polybasic carboxylic acid or alkyl ester thereof and polyhydric alcohol via direct esterification or trans-esterification. Examples of the polybasic carboxylic acid may include phthalic acid, isophthalic acid, terephthalic acid, tetrachlorophthalic acid, chlorophthalic acid, nitrophthalic acid, p- carboxyphenylacetic acid, p-phenylene-2-acetic acid, m-phenylenediglycolic acid, p-phenylenediglycolic acid, ophenylenediglycolic acid, diphenylacetic acid, diphenyl-p,pƍ-dicarboxylic acid, naphthalene-1,4-dicarboxylic acid, naphthalene- 1,5-dicarboxylic acid, naphthalene-2,6-dicarboxylic acid, anthracenedicarboxylic
86249859 acid, and/or cyclohexane dicarboxylic acid. Also, in addition to the dicarboxylic acid, a polybasic carboxylic acid such as trimellitic acid, pyromellitic acid, naphthalene tricarboxylic acid, naphthalene tetracarboxylic acid, pyrene tricarboxylic acid, and pyrene tetracarboxylic acid may be used. Also, derivatives of a carboxylic acid in which the carboxylic group thereof is reacted to form an anhydride, oxychloride, or ester group may be used. Among them, terephthalic acid or lower esters thereof, diphenyl acetic acid, cyclohexane di-carboxylic acid, or the like may be used. The lower ester refers to an ester of aliphatic alcohol having one to eight carbon atoms. Examples of the polyhydric alcohol may include an aliphatic diol such as ethylene glycol, diethylene glycol, triethylene glycol, propylene glycol, butane diol, hexane diol, neopentyl glycol, or glycerine, an alicyclic diol such as cyclohexane diol, cyclohexane dimethanol, or hydrogen- added bisphenol A, and an aromatic diol such as ethylene oxide adduct of bisphenol A or propylene oxide adduct of bisphenol A. One or more than one of the polyhydric alcohol may be used. Among these polyhydric alcohols, an aromatic diol and an alicyclic diol may be used. For example, an aromatic diol may be used. In addition, a polyhydric alcohol having three or more —OH groups, such as glycerin, trimethylol propane, or pentaerythritol may be used together with the diol to have a cross-linked structure or a branched structure to increase fixability or fusability of the toner. [0031] A number average molecular weight of the binder resin may be in the range of about 700 to about 1,000,000 g/ mole (mol) or about 10,000 to about 500,000 g/mol. The binder resin used in the present disclosure may include a combination of a high molecular weight binder resin and a low molecular weight binder resin in an appropriate ratio. A number average molecular weight of the high molecular weight binder resin may be, for example, from about 100,000 to about 500,000 g/mol, and a number average molecular weight of the low molecular weight binder resin may be, for example, from about 1,000 to about 100,000 g/mol. The two types of binder resins having different molecular weights may have independent functions. For instance, the low molecular weight binder resin has little molecular chain entanglements and may thereby contribute to fusability and gloss. On the contrary, the high molecular weight binder resin may
86249859 11 maintain a certain level of elasticity even at a high temperature due to many molecular chain entanglements and may thereby contribute to a higher hot offset occurring temperature. [0032] The pigment may be, for example, a black pigment, a yellow pigment, a magenta pigment, a cyan pigment, or any combination thereof. For example, the black pigment may be carbon black, aniline black, or any mixture thereof. [0033] For example, the yellow pigment may be a condensed nitrogen compound, an isoindolinon compound, an anthraquinone compound, an azo metal complex, an allyl imide compound, or any mixture thereof. More particularly, the yellow pigment may be, but is not limited to, “C.I. Pigment Yellow” 12, 13, 14, 17, 62, 74, 83, 93, 94, 95, 109, 110, 111, 128, 129, 147, 168, or 180. [0034] For example, the magenta pigment may be a condensed nitrogen compound, an anthraquinone compound, a quinacridone compound, a base dye lake, a naphthol compound, a benzoimidazole compound, a thioindigo compound, a perylene compound, or any mixture thereof. More particularly, the magenta pigment may be, but is not limited to, “C.I. Pigment Red” 2, 3, 5, 6, 7, 23, 48:2, 48:3, 48:4, 57:1, 81:1, 122, 144, 146, 166, 169, 177, 184, 185, 202, 206, 220, 221, or 254. [0035] For example, the cyan pigment may be a copper phthalocyanine compound or a derivative thereof, an anthraquinone compound, a base dye lake, or any mixture thereof. More particularly, the cyan pigment may be, but is not limited to, “C.I. Pigment Blue” 1, 7, 15, 15:1, 15:2, 15:3, 15:4, 60, 62, or 66. [0036] The amount of the pigment included in the toner particle may be, for example, from about 0.1 parts by weight to about 20 parts by weight, for example, from about 2 parts by weight to about 10 parts by weight, based on 100 parts by weight of the binder resin, without being limited thereto. [0037] Examples of the releasing agent may include, but are not limited to, a polyethylene-based wax, a polypropylene-based wax, a silicone-based wax, a paraffin-based wax, an ester-based wax, a carnauba-based wax, a metallocene- based wax, or any mixture thereof. [0038] The releasing agent may have, for example, a melting point from
86249859 about 50 °C to about 150 °C, without being limited thereto. The amount of the releasing agent included in the toner particle may be, for example, from about 1 part by weight to about 20 parts by weight, or from about 1 part by weight to about 10 parts by weight, based on 100 parts by weight of the binder resin. The releasing agent may prevent the toner particles from sticking to a heating roller of a fixing device. [0039] The toner particles may be prepared by, for example, a pulverization process, an aggregation process, or a spraying process. The pulverization process may be performed by, for example, pulverizing after melting and mixing a binder resin, a pigment, and a releasing agent. The aggregation process may be performed by, for example, mixing a binder resin dispersion, a pigment dispersion, and a releasing agent dispersion. The aggregation process may further include aggregating these particles of the binder resin, the pigment, and the releasing agent, and combining the resulting aggregates. [0040] A volume average particle diameter of the toner particles may be, but is not limited to, from about 4 micrometer (^m) to about 20 ^m or from about 5 ^m to about 8 ^m. [0041] A shape of the toner particles is also not particularly limited. As the shape of the toner particles is closer to a sphere, charging stability of the toner and dot reproducibility of a print image may be more enhanced. For example, the toner particles may have a sphericity in a range of, for example, about 0.90 to about 0.99. The sphericity of the toner particles can be measured by a flow particle image analyzer, FlowCam® 8000 manufactured by YOKOGAWA Corp. using an analysis software (VisualSpreadsheet® such as version 5.9.1.78). Specifically, in a 100 mL glass beaker, 0.1 mL to 0.5 mL of a surfactant, preferably Benzene, 1,1-oxybis, tetrapropylene derivatives, sulfonated, sodium salts (DOWFAX™ 2A1 manufactured by DOW Inc.) is loaded, approximately 0.1 g to 0.5 g of each toner is further added and stirred with a micro spatula, and then 80 ml of ion-exchanged water is added. Next, the obtained dispersion liquid is dispersed by an ultrasonic dispersion for about 3 minutes. The shape and distribution of the toner particles are measured using FlowCam® 8000, until the number of analyzed particles in the dispersion reaches 30000.
86249859 [0042] The method of forming the toner composition according to the present disclosure can include providing the toner particle, as provided herein and mixing the toner particle with the ultraviolet light active particle to attach the ultraviolet light active particle to the exterior surface of the toner particle. For example, the ultraviolet light active particles can be attached to the exterior surface of the toner particle to form the toner composition of the present disclosure by using a powder mixing apparatus without being limited thereto. Examples of the powder mixing apparatus may be, but are not limited to, a HENSCHEL Mixer®, a V shape mixer, a ball mill, or a NAUTA® mixer. [0043] The present disclosure can further include a kit for forming the toner composition. The kit can include a toner particle, where, as discussed herein, the toner particle includes a binder resin, a releasing agent, and a pigment and where the toner particle includes an exterior surface. The kit further includes the ultraviolet light active particle as discussed herein and a set of instructions detailing the steps for attaching the ultraviolet light active particle to the exterior surface of the toner particle. The set of instructions detailing the steps for attaching the ultraviolet light active particle to the exterior surface of the toner particle can include the method of forming the toner composition according to the present disclosure as discussed herein. [0044] As discussed herein, the toner composition includes the toner particle and one or more of the ultraviolet light active particles disposed on the exterior surface of the toner particle, where the toner composition absorbs ultraviolet light and emits visible light. In some examples, the ultraviolet light active particles are present on the exterior surface of the toner particle in a range from about 0.1 weight percent to about 3.0 weight percent based on the total weight of the toner composition. All Individual values and sub-ranges from 0.1 to 3.0 are included. For instance, in some examples the ultraviolet light active particles are present on the exterior surface of the toner particle at a value in a range having a lower value equal to 0.1, 0.5, or 1.0 to an upper value equal to 2.0, 2.5 or 3.0 weight percent based on the total weight of the toner composition, among other possibilities. [0045] The toner composition as provided herein absorbs long-wave
86249859 ultraviolet light in a range of about 315 nanometer (nm) to 400 nm and emits, in response to the absorbed long-wave ultraviolet light, visible light having a wavelength of about 400 nm to about 550 nm. In addition, the toner composition has a color difference value (¨B) of 10 to 20 using the CIELAB color space. Other properties of the toner composition as provided herein include having a relative intensity of emission of 0.5 to 3.0 as measured according to the procedure in the Examples section herein. [0046] In some examples, the ultraviolet light active particles can constitute 100 weight percent of a total weight of the additive added to the exterior surface of the toner particles. In some examples, the ultraviolet light active particles can constitute less than 100 weight percent of the total weight of the additive added to the exterior surface of the toner particles. For example, in some embodiments, other particles in addition to the ultraviolet light active particles can be disposed on a surface of the toner particles. Examples of such additional particles may include charge control agents and/or dispersing agents flow improvers among others. Examples of other additional particles may include but are not limited to titanium dioxide particles, sol-gel additives, aluminum oxide particles, strontium titanate particles, hydrophobic calcium carbonate particles, cerium oxide particles, silicon dioxide particles, tin oxide particles, zinc oxide particles, magnesium oxide particles, or any combination thereof. For instance, in some examples, the additional particles can be silica particles. The additional particles can have an average particle size in a range from about 10 nm to about 150 nm. [0047] The toner composition of the present disclosure is identifiable using ultraviolet light, as discussed herein, where the toner composition in either its power form or in its fused form (e.g., after electrostatic printing) responds by emitting visible light in the wavelengths provided herein. As such, the toner composition of the present disclosure can be identified without the need for additional processes and binding compounds for genuine product detection. In addition, it is a method that enables genuine product verification without other effects on printed images, especially color changes and printing performance. Furthermore, the life period of toner composition was improved, resulting from the reduction of drop-off additives on toner particles.
86249859 [0048] The toner compositions herein can be employed in an electrophotographic imaging apparatus which has a charging roller and/or an electrophotographic cartridge. Examples of electrophotographic imaging apparatus include a printer, a copier, a scanner, a fax machine, or a multifunction peripheral incorporating two or more of these. [0049] The electrophotographic imaging apparatus may include an electrophotographic cartridge. The electrophotographic cartridge may include an electrophotographic photoconductor drum that is charged by a charging roller according to an example, which is a charging means disposed in contact with the electrophotographic photoconductor drum. The electrophotographic photoconductor drum may be rotationally driven at a predetermined circumferential speed about an axis. The electrophotographic photoconductor drum may be subjected to uniform charging of a positive or a negative predetermined potential on its surface by the charging roller in the rotation process. The voltage applied to the charging roller may be, for example, a DC voltage. However, the voltage applied to the charging roller may be, for example, a combination of an AC voltage and a DC voltage. In the electrophotographic imaging apparatus according to an example, even when a DC voltage is applied to the charging roller, stable charging characteristics may be maintained for a longer period of time, and a high-quality output image may be obtained. [0050] The charging roller may charge the surface of the electrophotographic photoconductor drum to a uniform potential value while rotating in contact with the electrophotographic photoconductor drum. The image portion is exposed by laser light to form an electrostatic latent image on the electrophotographic photoconductor drum. After the electrostatic latent image is made a visible image, for example, a toner image, by a developing unit, the toner image is transferred to an image receiving member such as paper by a transfer unit such as the transfer roller to which a voltage is applied. Toner remaining on the surface of the electrophotographic photoconductor drum after the image transfer is cleaned by a cleaning unit, for example, a cleaning blade. The electrophotographic photoconductor drum may be used again for image formation. The developing unit includes a regulating blade, a developing roller, and a supply
86249859 roller. [0051] The electrophotographic cartridge according to an example may integrally support the electrophotographic photoconductor drum, the charging roller, and the cleaning blade, may be attached to the electrophotographic imaging apparatus, and may be detached from the electrophotographic imaging apparatus. Another cartridge may integrally support the developing unit including the regulating blade, the developing roller, and the supply roller, may be attached to the electrophotographic imaging apparatus, and may be detached from the electrophotographic imaging apparatus. Toner compositions such as those described herein (e.g., toner) may be located inside the developing unit. [0052] Examples [0053] Hereinafter, various examples will be described. However, the scope of the disclosure is not limited thereto. [0054] The types and properties of the ultraviolet light active particles and the toner composition used in Examples (EX) 1 to 8 and Comparative Examples (CE) A to H are described below. Formation of Ultraviolet Light Active Particles EX 1 to EX 4 and Particles of CE A to CE D: Ultraviolet Light Active Particle of EX 1 and EX 2 – Polystyrene [0055] A synthesis method of the ultraviolet light active particle, where the first polymer of the core is formed using polystyrene (PS) and an ultraviolet light active compound, according to the present disclosure. To form the core, 1 kilogram (kg) of distilled water was added to a 2 liter (L) reactor, heated to an internal temperature of 80 °C under a nitrogen atmosphere, and then a mixed solution of 275 gram (g) of styrene monomer, 16 g of ethyl acrylate, 111 g of allyl methacrylate, 10 g of 6-methoxy-2-(octan-3-yl)-1H-benzo[de]isoquinoline- 1,3(2H)-dione and 14 g of sodium dioctyl sulforsuccinate was stirred in the reactor for 100 minutes. Thereafter, 3 g of a 2.5 wt.% potassium persulfate solution was introduced into the reactor and emulsified and polymerized for 60 minutes to
86249859 prepare the core. [0056] To form the shell, a mixed solution of 218 g of styrene monomer, 11 g of ethyl acrylate, 1 g of dodecylmercaptane, and 0.5 g of azobisisobutyronitrile was added to the reactor including the core-shell type emulsion at 50 °C. After the temperature was gradually increased to 80 °C, polymerization was performed for 120 minutes to form a protective layer on the shell layer, and then polymer composite, which are final products in powder form, were manufactured using the salting out method, as described herein. Ultraviolet Light Active Particle of EX 3 and EX 4 – Polymethyl Methacrylate [0057] A synthesis method of the ultraviolet light active particle, where the first polymer of the core is formed using polymethyl methacrylate (PMMA) and an ultraviolet light active compound, according to the present disclosure. To form the core, 1 kilogram (kg) of distilled water was added to a 2 liter (L) reactor, heated to an internal temperature of 80 °C under a nitrogen atmosphere, and then a mixed solution of 275 g of methyl methacrylate, 16 g of ethyl acrylate, 111 g of allyl methacrylate, 10 g of 6-methoxy-2-(octan-3-yl)-1H-benzo[de]isoquinoline- 1,3(2H)-dione and 14 g of sodium dioctyl sulforsuccinate was stirred in the reactor for 100 minutes. Thereafter, 3 g of a 2.5 wt.% potassium persulfate solution was introduced into the reactor and emulsified and polymerized for 60 minutes to prepare the core. [0058] To form the shell, a mixed solution of 218 g of methyl methacrylate, 11 g of ethyl acrylate, 1 g of dodecylmercaptane, and 0.5 g of azobisisobutyronitrile was added to the reactor including the core-shell type emulsion at 50 °C. After the temperature was gradually increased to 80 °C, polymerization was performed for 120 minutes to form a protective layer on the shell layer, and then polymer composite, which are final products in powder form, were manufactured using the salting out method, as described herein. Particles of CE A – CE D [0059] CE A was prepared as follows. To form the core, 1 kg of distilled water was added to a 2 L reactor, heated to an internal temperature of 80 °C
86249859 under a nitrogen atmosphere, and then a mixed solution of 275 g of styrene monomer, 16 g of ethyl acrylate, 111 g of allyl methacrylate and 14 g of sodium dioctyl sulforsuccinate was stirred in the reactor for 100 minutes. Thereafter, 3 g of a 2.5 wt.% potassium persulfate solution was introduced into the reactor and emulsified and polymerized for 60 minutes to prepare the core. [0060] To form the shell, a mixed solution of 218 g of styrene monomer, 11 g of ethyl acrylate, 1 g of dodecylmercaptane, and 0.5 g of azobisisobutyronitrile was added to the reactor including the core-shell type emulsion at 50 °C. After the temperature was gradually increased to 80 °C, polymerization was performed for 120 minutes to form a protective layer on the shell layer, and then polymer composite, which are final products in powder form, were manufactured using the salting out method, as described herein. [0061] CE B and CE D were each the ultraviolet light active compound 6- methoxy-2-(octan-3-yl)-1H-benzo[de]isoquinoline-1,3(2H)-dione as provided in Table 1. [0062] CE C was prepared as follows. To form the core, 1 kg of distilled water was added to a 2 L reactor, heated to an internal temperature of 80 °C under a nitrogen atmosphere, and then a mixed solution of 275 g of methyl methacrylate, 16 g of ethyl acrylate, 111 g of allyl methacrylate, and 14 g of sodium dioctyl sulforsuccinate was stirred in the reactor for 100 minutes. Thereafter, 3 g of a 2.5 wt.% potassium persulfate solution was introduced into the reactor and emulsified and polymerized for 60 minutes to prepare the core. [0063] To form the shell, a mixed solution of 218 g of methyl methacrylate, 11 g of ethyl acrylate, 1 g of dodecylmercaptane, and 0.5 g of azobisisobutyronitrile was added to the reactor including the core-shell type emulsion at 50 °C. After the temperature was gradually increased to 80 °C, polymerization was performed for 120 minutes to form a protective layer on the shell layer, and then polymer composite, which are final products in powder form, were manufactured using the salting out method, as described herein. Table 1 – EX and CE of Ultraviolet Light Active Particle

86249859

Relative Intensity of Emission [0064] Relative intensity of emission (maximum wavelength of absorption and emission UV absorb, Vis fluorescence) was measured using a Scinco S-3100 spectrophotometer for UV-vis and Scinco FS-2 spectrophotometer for Fluorescence. The detailed measurement method was obtained by dissolving the EX or CE in an organic solvent (acetone, ethanol or others) in an amount of 0.01 wt.%, putting it in a measurement cell, and then measuring the properties. A wavelength of 440 nm was used for the excitation for each of the EX and CE. Sphericity of the Ultraviolet Light Active Particles [0065] The sphericity of the ultraviolet light active particles was determined by visual observation with a scanning electron microscope (Hitachi S-4800 SEM). Toner Particle [0066] Synthesis method of black synthesized toner powder. A method of preparing the black synthesized toner is as follows. A latex mixture, a black pigment dispersion solution, and a wax dispersion solution are mixed in the reactor. Agglomerating agents are mixed in this mixture and stirred sufficiently using a homogenizer. Thereafter, an additional latex mixture is additionally added and stirred. Next, after the emulsion-aggregation process, a black toner can be obtained through cooling, filtering, drying, and grinding processes.
86249859 [0067] A method of preparing the black pulverized toner is as follows. A polymer resin, a black pigment, release agent, and charge control agent are mixed in the mixer. The mixture was melted and kneaded using a twin-screw extruder. After being cooled on a cooling belt, the resultant melt-kneaded product was coarsely pulverized by means of a speed mill having a 1-mm-diameter screen, finely pulverized by means of a jet pulverizer, and further classified with an Elbow- Jet classifier, thereby producing toner base particle. Preparation Toner Composition of EX 5 – EX 8 and Toner CE E – CE H [0068] For each of the toner compositions of EX 5 – EX 8 and CE E – CE H seen in Table 2, 100 parts by weight of toner particle was injected into an additive mixer (Daehwa Tech, Korea, KM-LS2K), and then 0.5 parts by weight of a fumed type silica and 1.0 parts by weight of a sol-gel type silica was added for EX 1 – EX 4 or CE A – CE D, as seen in Table 1, according to the amounts seen in Table 2. The mixture was mixed at about 2,000 rpm for 30 seconds in a 2 L mixer, and further mixed at about 6,000 rpm for 3 minutes to prepare toner particles of EX 5 – EX 8 and CE E – CE H. Table 2 – Toner Composition of EX 5 – EX 8 and Toner CE E – CE H
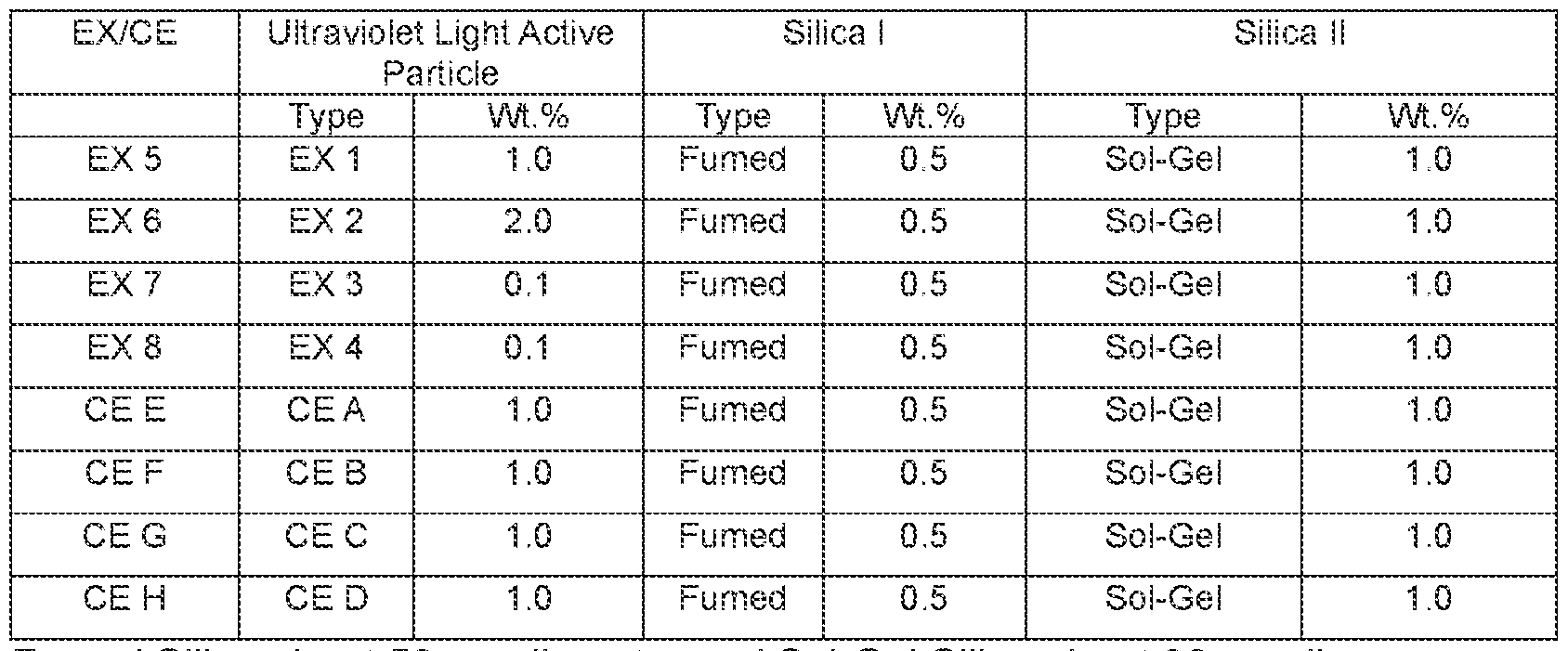
Fumed Silica about 50 nm diameter and Sol-Gel Silica about 30 nm diameter Image Background Analysis - Optical Photo Conductor (OPC) Background Performance: [0069] OPC was used to detect and analyze features or characteristic
86249859 21 points in the background of a printed image. Since the background image is generated under printing conditions with high temperature/high humidity, the above test was conducted under high temperature/high humidity conditions. After each of the toner compositions of the examples and comparative examples were loaded into a toner cartridge of a two-component development system printer (MFP E826, available from HP®), images having a resolution of approximately 7,000 pixels in the horizontal dimension (“7K images”) with 2% coverage were printed at 32 °C and 80% humidity. An average image background was determined by measuring the optical density reading at three non-image locations of the OPC drum. Each optical density reading was measured using an "ELECTROEYE" reflection densitometer. Image background performance was classified according to the following criteria. [0070] ^: less than 0.02 optical density (the toner composition has very good OPC background performance). [0071] ż: optical density of 0.02 or more and less than 0.03 (the toner composition has excellent OPC background performance). [0072] ^: optical density of 0.03 or more and less than 0.05 (the toner composition has poor OPC background performance). [0073] ×: optical density of 0.05 or more (the toner composition has very poor OPC background performance). Color Difference Value (¨B) Using CIELAB Color Space [0074] The color coordinates of the toner composition EX and CE were measured using an X-Rite® eXact™ Advanced spectrophotometer. The detailed analysis method was obtained through an X-Rite® eXact™ spectrophotometer by evenly spreading 2 g of the EX or CE on a filter paper and then measuring and printing photos with a digital camera. The UV irradiation was obtained through an analytical device by using a UV lamp device and then photographing and printing with a digital camera.
86249859 Table 3 – Toner Composition EX 5 – EX 98 and Toner CE E – CE H

Color Difference Value - ¨ B (|b*V - b*uv|) : |b* value on visible light – b* value on UV light using the CIELAB color space. [0075] In Table 3, it can be seen that the small ¨ B values were shown in CE E and CE G due to the absence of the ultraviolet light active particles in toner composition, resulting in failure of identification between a genuine toner and a counterfeit toner caused by the lower value than the desired threshold value. Although CE F and CE H appeared to have larger ¨ B values, the performance of the image background deteriorated due to the low circularity and the large size of the ultraviolet light active compound itself. [0076] Although examples of the disclosure have been illustrated and described hereinabove, the disclosure is not limited thereto, and may be variously modified and altered by those skilled in the art to which the disclosure pertains without departing from the disclosure claimed in the claims. These modifications and alterations are to fall within the scope of the disclosure. As used herein the term “about” refers to value(s) that are within 10 percent, within 5 percent or within 1 percent of a given value that the term about modifies. For instance, the term about can refer to a value(s) that are within 10 percent (+/- 10 percent) of a given value.