BENZIMIDAZOLE DERIVATIVES The present invention relates to microbiocidal benzimidazole derivatives, e.g. as active ingredients, which have microbiocidal activity, in particular fungicidal activity, more particularly activity against oomycetes. The invention also relates to preparation of these benzimidazole derivatives, to intermediates useful in the preparation of these benzimidazole derivatives, to the preparation of these intermediates, to agrochemical compositions which comprise at least one of the benzimidazole derivatives, to preparation of these compositions and to the use of the benzimidazole derivatives or compositions in agriculture or horticulture for combating, controlling or preventing infestation of plants, harvested food crops, seeds or non-living materials by phytopathogenic microorganisms, in particular fungi, more particularly oomycetes. It has now surprisingly been found that certain novel benzimidazole derivatives have favourable fungicidal properties, in particular against oomycetes. Therefore, in a first aspect, the present invention provides compounds of formula (I)
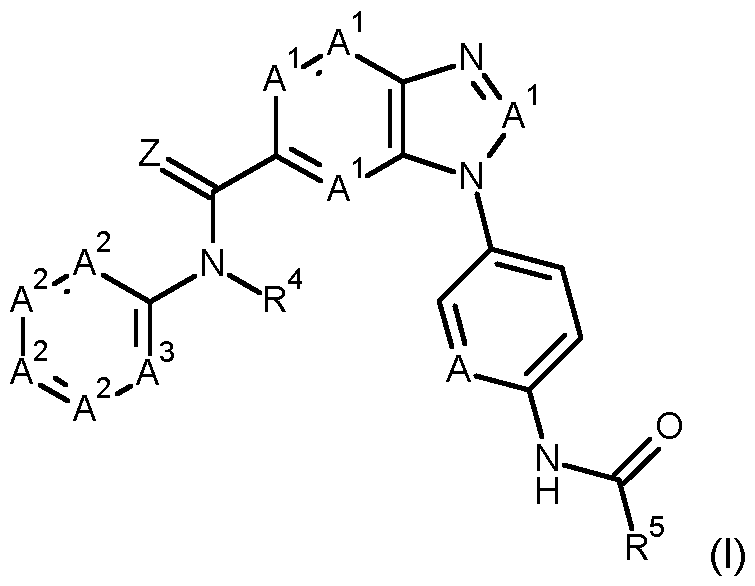
wherein Z is O or S, and preferably Z is O; A is CH or N; A
1 are independently N or CR
1; with the proviso that no more than three A
1 are N, preferably no more than two A
1 are N, preferably no more than one A
1 is N, and more preferably the four A
1 are CR
1; R
1 are independently selected from hydrogen, hydroxy, halogen, CN, C1-6alkyl, C3-6cycloalkyl, C1- 6alkoxy-C1-6alkyl, C3-6cycloalkyl-C1-4alkyl, C1-6alkylsulfanyl, C1-6alkylsulfinyl, C1-6alkylsulfonyl, C1-6alkoxy, amino, and NHC(O)C1-6alkyl; A
2 are independently CR
2 or N, with the proviso that no more than three A
2 are N, preferably no more than two A
2 are N, preferably no more than one A
2 is N, and more preferably the four A
2 are CR
2; R
2 are independently selected from hydrogen, hydroxy, halogen, CN, C1-6alkyl, C1-6alkoxy, C1-6alkoxy- C1-6alkyl, C1-6alkoxy-C1-6alkoxy, C2-6alkenyl, C2-6alkynyl, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, C1- 6alkylsulfanyl, C1-6alkylsulfinyl, C1-6alkylsulfonyl, C1-6alkoxycarbonyl, C1-6alkylaminocarbonyl, diC1-
6alkylaminocarbonyl, and C1-6alkylcarbonyl, wherein each of the C1-6alkyl, C1-6alkoxy, C1-6alkoxy-C1- 6alkyl, C1-6alkoxy-C1-6alkoxy, C2-6alkenyl, C2-6alkynyl, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, C1- 6alkylsulfanyl, C1-6alkylsulfinyl, C1-6alkylsulfonyl, C1-6alkoxycarbonyl, C1-6alkylaminocarbonyl, diC1- 6alkylaminocarbonyl, and C1-6alkylcarbonyl groups is optionally substituted with one to three substituents independently selected from halogen, hydroxy, and CN; A
3 is independently CR
3 or N; R
3 are independently selected from hydrogen, hydroxy, halogen, CN, C1-6alkyl, C1-6alkoxy, C1-6alkoxy- C1-6alkyl, C1-6alkoxy-C1-6alkoxy, C2-6alkenyl, C2-6alkynyl, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, C1- 6alkylsulfanyl, C1-6alkylsulfinyl, C1-6alkylsulfonyl, amino, C1-6alkylamino, diC1-6-alkylamino, and C3- 6cycloalkylamino, wherein each of the C1-6alkyl, C1-6alkoxy, C1-6alkoxy-C1-6alkyl, C1-6alkoxy-C1-6alkoxy, C2-6alkenyl, C2-6alkynyl, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, C1-6alkylsulfanyl, C1-6alkylsulfinyl, C1- 6alkylsulfonyl, amino, C1-6alkylamino, diC1-6-alkylamino, and C3-6cycloalkylamino groups is optionally substituted with one to three substituents independently selected from halogen, hydroxy, and CN; R
4 is selected from C1-6alkyl, C1-6alkoxy-C1-6alkyl, C3-6cycloalkyl, C3-6cycloalkyl-C1-4alkyl, C2-6alkenyl, C2- 6alkynyl, C1-6alkoxy, C1-6alkylsulfanyl-C1-6alkyl, C1-6alkylsulfinyl-C1-6alkyl, C1-6alkylsulfonyl-C1-6alkyl, C1- 6alkoxycarbonyl-C1-6alkyl, C1-6alkylaminocarbonyl-C1-6alkyl, diC1-6alkylaminocarbonyl-C1-6alkyl, and CN, wherein each of the C1-6alkyl, C1-6alkoxy-C1-6alkyl, C3-6cycloalkyl, C3-6cycloalkyl-C1-4alkyl, C2-6alkenyl, C2- 6alkynyl, C1-6alkoxy, C1-6alkylsulfanyl-C1-6alkyl, C1-6alkylsulfinyl-C1-6alkyl, C1-6alkylsulfonyl-C1-6alkyl, C1- 6alkoxycarbonyl-C1-6alkyl, C1-6alkylaminocarbonyl-C1-6alkyl and diC1-6alkylaminocarbonyl-C1-6alkyl groups is optionally substituted with one to three substituents independently selected from halogen and CN; wherein A
3 and R
4 taken together optionally form a ring, more preferably a 5-8-membered heterocycle, more preferably a 6-membered heterocycle; and R
5 is selected from C1-6alkyl, C1-6alkoxy, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, C1-6alkoxyC1-6alkyl, C1- 6alkylamino, diC1-6alkylamino, and C1-6alkylC1-6alkoxyamino, wherein each of said groups is optionally substituted with one to three substituents independently selected from halogen and CN; or a salt or N-oxide thereof. In a second aspect the present invention provides an agrochemical composition comprising a compound of formula (I), and more particularly an agrochemical composition comprising a fungicidally effective amount of a compound of formula (I). Said composition can further comprise at least one compound selected among an additional active ingredient, an appropriate formulation inert, a carrier, an adjuvant, and any mixtures thereof. Compounds of formula (I) may be used to control phytopathogenic microorganisms. Thus, in order to control a phytopathogen a compound of formula (I), or a composition comprising a compound of formula (I) according to the invention, may be applied directly to the phytopathogen, to the locus of a
phytopathogen, in particular to a plant susceptible to attack by phytopathogens, or to a propagation material of a plant. Thus, in a third aspect the present invention provides the use of a compound of formula (I), or a composition comprising a compound of formula (I), as described herein to combat, prevent or control a phytopathogen. In a fourth aspect the present invention provides a method of combating, preventing or controlling phytopathogens, comprising applying a compound of formula (I), or a composition comprising a compound of formula (I), as described herein to said phytopathogen, to the locus of said phytopathogen, in particular to a plant susceptible to attack by a phytopathogen, or to a propagation material of a plant. Compounds of formula (I) are particularly effective in combating, preventing or controlling phytopathogenic fungi, in particular oomycetes. Thus, in a fifth aspect the present invention provides the use of a compound of formula (I), or a composition comprising a compound of formula (I), as described herein to control phytopathogenic fungi, in particular oomycetes. In a sixth aspect the present invention provides a method of combating, preventing or controlling phytopathogenic disease, such as phytopathogenic fungi, comprising applying a compound of formula (I), or a composition comprising a compound of formula (I), as described herein to said phytopathogenic fungi, or to the locus of said phytopathogenic fungi, in particular to a plant susceptible to attack by phytopathogenic fungi, in particular oomycetes, or to a propagation material of a plant. Where a group is indicated as being substituted, e.g. alkyl, this includes those groups that are part of other groups, e.g. the alkyl in alkylthio. Definitions: - The term "halogen" refers to fluorine (fluoro or F), chlorine (chloro or Cl), bromine (bromo or Br) or iodine (iodo or I), preferably fluorine, chlorine or bromine. - The term "Alkyl" as used herein- in isolation or as part of a chemical group – represents straight-chain or branched hydrocarbons, preferably with 1 to 6 carbon atoms, for example methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, s-butyl, t-butyl, pentyl, 1- methylbutyl, 2-methylbutyl, 3-methylbutyl, 1,2- dimethylpropyl, 1,1 -dimethylpropyl, 2,2- dimethylpropyl, 1 -ethylpropyl, hexyl, 1 -methylpentyl, 2- methylpentyl, 3-methylpentyl, 4- methylpentyl, 1,2-dimethylpropyl, 1,3-dimethylbutyl, 1,4-dimethylbutyl, 2,3-dimethylbutyl, 1,1- dimethylbutyl, 2,2-dimethylbutyl, 3,3-dimethylbutyl, 1,1,2-trimethylpropyl, 1,2,2- trimethylpropyl, 1- ethylbutyl and 2-ethylbutyl. Alkyl groups with 1 to 4 carbon atoms are preferred, for example methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, s-butyl or t-butyl. - The term "Alkenyl" - in isolation or as part of a chemical group - represents straight-chain or branched hydrocarbons, preferably with 2 to 6 carbon atoms and at least one double bond, for example vinyl, 2- propenyl, 2-butenyl, 3-butenyl, 1- methyl-2-propenyl, 2-methyl-2-propenyl, 2-pentenyl, 3-pentenyl, 4-
pentenyl, 1-methyl-2-butenyl, 2- methyl-2-butenyl, 3-methyl-2-butenyl, 1-methyl-3-butenyl, 2-methyl-3- butenyl, 3-methyl-3-butenyl, 1,1 - dimethyl-2-propenyl, 1,2-dimethyl-2-propenyl, 1 -ethyl-2-propenyl, 2- hexenyl, 3-hexenyl, 4- hexenyl, 5-hexenyl, 1 -methyl-2-pentenyl, 2-methyl-2-pentenyl, 3-methyl-2- pentenyl, 4-methyl-2- pentenyl, 3-methyl-3-pentenyl, 4-methyl-3-pentenyl, 1 -methyl-4-pentenyl, 2- methyl-4-pentenyl, 3- methyl-4-pentenyl, 4-methyl-4-pentenyl, 1, 1 -dimethyl-2-butenyl, 1,1-dimethyl-3- butenyl, 1,2- dimethyl-2-butenyl, l,2-dimethyl-3-butenyl, 1,3-dimethyl-2-butenyl, 2,2-dimethyl-3-butenyl, 2,3- dimethyl-2-butenyl, 2,3-dimethyl-3-butenyl, 1 -ethyl-2-butenyl, 1-ethyl-3-butenyl, 2-ethyl-2-butenyl, 2-ethyl-3-butenyl, 1, 1,2-trimethyl-2-propenyl, 1 -ethyl- 1 -methyl-2-propenyl und 1-ethyl-2-methyl-2- propenyl. Alkenyl groups with 2 to 4 carbon atoms are preferred, for example 2-propenyl, 2-butenyl or 1-methyl-2-propenyl. - The term "Alkynyl" - in isolation or as part of a chemical group - represents straight-chain or branched hydrocarbons, preferably with 2 to 6 carbon atoms and at least one triple bond, for example 2-propynyl, 2-butynyl, 3-butynyl, 1-methyl-2- propynyl, 2-pentynyl, 3-pentynyl, 4-pentynyl, 1-methyl-3-butynyl, 2- methyl-3-butynyl, 1-methyl-2- butynyl, 1,1 -dimethyl-2-propynyl, 1 -ethyl-2-propynyl, 2-hexynyl, 3- hexynyl, 4-hexynyl, 5-hexynyl, 1- methyl-2-pentynyl, 1-methyl-3-pentynyl, 1 -methyl-4-pentynyl, 2- methyl-3-pentynyl, 2-methyl-4- pentynyl, 3 -methyl-4-pentynyl, 4-methyl-2-pentynyl, 1,1 -dimethyl-3 - butynyl, 1,2-dimethyl-3 –butynyl, 2,2- dimethyl-3-butynyl, 1-ethyl-3-butynyl, 2-ethyl-3-butynyl, 1-ethyl-1- methyl-2-propynyl and 2,5-hexadiynyl. Alkynyls with 2 to 4 carbon atoms are preferred, for example ethynyl, 2- propynyl or 2-butynyl-2-propenyl. - The term "cycloalkyl" - in isolation or as part of a chemical group - represents saturated or partially unsaturated mono-, bi- or tricyclic hydrocarbons, preferably with 3 to 10 carbon atoms, for example cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, bicyclo[2.2.1]heptyl, bicyclo[2.2.2]octyl or adamantyl. Cycloalkyls with 3, 4, 5, 6 or 7 carbon atoms are preferred, for example cyclopropyl or cyclobutyl. - The term “alkoxy" refers to a radical of the formula -ORa wherein Ra is an alkyl radical as generally defined above. Examples of alkoxy include, but are not limited to methoxy, ethoxy, propoxy, iso-propoxy, and tert-butoxy. - The term “ alkylsulfanyl” refers to a radical of the formula -SRa wherein Ra is an alkyl radical as generally defined above. - The term “alkylsulfinyl” refers to a radical of the formula -S(O)Ra wherein Ra is an alkyl radical as generally defined above. - The term “alkylsulfonyl” refers to a radical of the formula -S(O)2Ra wherein Ra is an alkyl radical as generally defined above. - the term “alkoxycarbonyl” refers to a radical of the formula RaOC(O)-, wherein Ra is an alkyl radical as generally defined above.
- The term “alkylaminocarbonyl” refers to a radical of the formula RaNHC(O)- wherein Ra is an alkyl radical as generally defined above. - Hydroxyl or hydroxy stands for a –OH group. The term ”combating”, “preventing” or “controlling”, and its inflections, within the context of the present invention, mean reducing any undesired effect, such as pathogenic and more particularly phytopathogenic, especially fungi such as oomycetes, infestation or attack of, and pathogenic damage to a plant or to a plant derived product to such a level that an improvement is demonstrated. As used herein, the term "effective amount" refers to the amount of the compound, a salt, or N-oxide thereof, which, upon single or multiple applications provides the desired effect. An effective amount is readily determined by the skilled person in the art, by the use of known techniques and by observing results obtained under analogous circumstances. In determining the effective amount a number of factors are considered including, but not limited to: the type of plant or derived product to be applied; the pathogen to be controlled & its lifecycle; the particular compound applied; the type of application; and other relevant circumstances. Compounds of formula (I) which have at least one basic centre can form, for example, acid addition salts, for example with strong inorganic acids such as mineral acids, for example perchloric acid, sulfuric acid, nitric acid, nitrous acid, a phosphorus acid or a hydrohalic acid, with strong organic carboxylic acids, such as C
1-C
4alkanecarboxylic acids which are unsubstituted or substituted, for example by halogen, for example acetic acid, such as saturated or unsaturated dicarboxylic acids, for example oxalic acid, malonic acid, succinic acid, maleic acid, fumaric acid or phthalic acid, such as hydroxycarboxylic acids, for example ascorbic acid, lactic acid, malic acid, tartaric acid or citric acid, or such as benzoic acid, or with organic sulfonic acids, such as C1-C4alkane- or arylsulfonic acids which are unsubstituted or substituted, for example by halogen, for example methane- or p-toluenesulfonic acid. Compounds of formula (I) which have at least one acidic group can form, for example, salts with bases, for example mineral salts such as alkali metal or alkaline earth metal salts, for example sodium, potassium or magnesium salts, or salts with ammonia or an organic amine, such as morpholine, piperidine, pyrrolidine, a mono-, di- or tri-lower-alkylamine, for example ethyl-, diethyl-, triethyl- or dimethylpropylamine, or a mono-, di- or trihydroxy-lower-alkylamine, for example mono-, di- or triethanolamine. In each case, the compounds of formula (I) according to the invention are in free form, in oxidized form as an N-oxide, in covalently hydrated form, or in salt form, e.g., an agronomically usable or agrochemically acceptable salt form. N-oxides are oxidized forms of tertiary amines or oxidized forms of nitrogen containing heteroaromatic compounds. They are described for instance in the book “Heterocyclic N-oxides” by A. Albini and S. Pietra, CRC Press, Boca Raton 1991. The compounds of formula (I) according to the invention also include hydrates, which may be formed during salt formation.
The compounds of formula (I) according to the invention also include hydrates which may be formed during the salt formation. In a further embodiment, there is provided a compound of formula (I) according to the present invention, wherein the four A
1 are CR
1. In a further embodiment, there is provided a compound of formula (I) according to the present invention, wherein two A
1 are CR
1 and two A
1 are N. In a further embodiment, there is provided a compound of formula (I) according to the present invention, wherein three A
1 are CR
1 and one A
1 is N. In a further embodiment, there is provided a compound of formula (I) according to the present invention, wherein R
1 are independently selected from hydrogen, hydroxy, halogen, CN, C1-6alkyl, C3-6cycloalkyl, C
1-6alkoxy-C
1-6alkyl, C
3-6cycloalkyl-C
1-4alkyl, C
1-6alkoxy, amino, and NHC(O)C
1-6alkyl, and preferably R
1 are independently selected from hydrogen and C1-6alkyl. In a further embodiment, there is provided a compound of formula (I) according to the present invention, wherein
. In a further embodiment, there is provided a compound of formula (I) according to the present invention, wherein R
2 are independently selected from hydrogen, hydroxy, halogen, CN, C1-6alkyl, C1-6alkoxy, C1- 6alkoxy-C1-6alkyl, and C1-6alkoxy-C1-6alkoxy, C1-6alkoxycarbonyl, C1-6alkylaminocarbonyl, diC1-
6alkylaminocarbonyl, and C
1-6alkylcarbonyl, wherein each of the C
1-6alkyl, C
1-6alkoxy, C
1-6alkoxy-C
1- 6alkyl, C1-6alkoxy-C1-6alkoxy, C1-6alkoxycarbonyl, C1-6alkylaminocarbonyl, diC1-6alkylaminocarbonyl, and C1-6alkylcarbonyl groups is optionally substituted with one to three substituents independently selected from halogen, hydroxy, and CN; and preferably R
2 are independently selected from hydrogen, halogen, CN, C1-6alkyl, C1-6alkoxy, C1-6alkoxy-C1-6alkyl, and C1-6alkoxy-C1-6alkoxy, wherein each of the C1-6alkyl, C1-6alkoxy, C1-6alkoxy-C1-6alkyl, and C1-6alkoxy-C1-6alkoxy groups is optionally substituted with one to three substituents independently selected from halogen, hydroxy, and CN.
In a further embodiment, there is provided a compound of formula (I) according to the present invention, wherein R
3 are independently selected from hydrogen, hydroxy, halogen, CN, C1-6alkyl, C1-6alkoxy, C1- 6alkoxy-C1-6alkyl, C1-6alkoxy-C1-6alkoxy, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, amino, C1-6alkylamino, diC1-6-alkylamino, and C3-6cycloalkylamino, wherein each of the C1-6alkyl, C1-6alkoxy, C1-6alkoxy-C1- 6alkyl, C1-6alkoxy-C1-6alkoxy, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, amino, C1-6alkylamino, diC1-6- alkylamino and C3-6cycloalkylamino groups is optionally substituted with one to three substituents independently selected from halogen, hydroxy, and CN. In a preferred embodiment, R
3 can be hydrogen. In a further embodiment, there is provided a compound of formula (I) according to the present invention, wherein four A
2 are CR
2 and A
3 is N. In a further embodiment, there is provided a compound of formula (I) according to the present invention, wherein
, and preferably the three A
2 are CR
2 and A
3 is CR
3. In a further embodiment, there is provided a compound of formula (I) according to the present invention, wherein
preferably the three A
2 are CR
2 and A
3 is CR
3. In a further embodiment, there is provided a compound of formula (I) according to the present invention, wherein four A
2 are CR
2 and A
3 is CR
3, and preferably
. In the particular embodiment wherein
, R
2 is as defined in the present invention; preferably R
2 are independently selected from hydrogen, hydroxy, halogen, CN, C1-6alkyl, C1-6alkoxy, C1-6alkoxy-C1-6alkyl, C1-6alkoxy-C1-6alkoxy, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, C1-6alkoxycarbonyl, C1-6alkylaminocarbonyl, diC1-6alkylaminocarbonyl, and C1-6alkylcarbonyl, wherein each of the C1-6alkyl, C1-6alkoxy, C1-6alkoxy-C1-6alkyl, C1-6alkoxy-C1-6alkoxy, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, C1- 6alkoxycarbonyl, C1-6alkylaminocarbonyl, diC1-6alkylaminocarbonyl, and C1-6alkylcarbonyl groups is optionally substituted with one to three substituents independently selected from halogen, hydroxy, and
CN; and more preferably R
2 are independently selected from hydrogen, halogen, CN, C1-6alkoxy, C1- 6alkoxy-C1-6alkyl, and C1-6alkoxy-C1-6alkoxy, wherein each of the C1-6alkoxy, C1-6alkoxy-C1-6alkyl, and C1-6alkoxy-C1-6alkoxy groups is optionally substituted with one to three substituents independently selected from halogen, hydroxy, and CN. In a further embodiment, there is provided a compound of formula (I) according to the present invention, wherein R
4 is selected from C
1-6alkyl, C
1-6alkoxy-C
1-6alkyl, C
3-6cycloalkyl, C
3-6cycloalkyl-C
1-4alkyl, C
2- 6alkenyl, C2-6alkynyl, and C1-6alkoxy, wherein each of the C1-6alkyl, C1-6alkoxy-C1-6alkyl, C3-6cycloalkyl, C3-6cycloalkyl-C1-4alkyl, C2-6alkenyl, C2-6alkynyl, and C1-6alkoxy groups is optionally substituted with one to three substituents independently selected from halogen and CN; preferably and R
4 is selected from C1-6alkyl, C1-6alkoxy-C1-6alkyl, C3-6cycloalkyl, C3-6cycloalkyl-C1-4alkyl, and C1-6alkoxy, wherein each of the C1-6alkyl, C1-6alkoxy-C1-6alkyl, C3-6cycloalkyl, C3-6cycloalkyl-C1-4alkyl, and C1-6alkoxy groups is optionally substituted with one to three substituents independently selected from halogen and CN; and wherein A
3 and R
4 taken together optionally form a ring, more preferably a 5-8-membered heterocycle, more preferably a 6-membered heterocycle. In a further embodiment, there is provided a compound of formula (I) according to the present invention, wherein A
3 is CR
3 and wherein R
3 and R
4 taken together form a ring, preferably a 5-8-membered heterocycle, preferably a 6-membered heterocycle, and more preferably one of the rings W1, W2 or W3 as described in the compounds of the formula (I) below:
The carbon and/or the nitrogen atoms forming said ring (W1, W2 or W3) can be substituted according to the R
3 and R
4 groups as defined in the present invention, and more preferably can be substituted by a R
3’ group, wherein R
3’ is selected from hydrogen, C1-6alkyl, and C3-6cycloalkyl, wherein each of the C1- 6alkyl and C3-6cycloalkyl groups is optionally substituted with one to three substituents independently selected from halogen and CN. For example, the compounds of the formula (I-W3) can be as follows:
In a preferred embodiment, the compounds of the formula (I-W1), (I-W2) and (I-W3) can be as described below:
The carbon and/or the nitrogen atoms forming said ring (W1, W2 or W3) can be substituted, especially by a R
3’ group, wherein R
3’ is selected from hydrogen, C1-6alkyl, and C3-6cycloalkyl, wherein each of the C1-6alkyl and C3-6cycloalkyl groups is optionally substituted with one to three substituents independently selected from halogen and CN. For example, the compounds of the formula (I-W3) can be as follows:
In a further embodiment, there is provided a compound of formula (I) according to the present invention, wherein R
5 is selected from C1-6alkyl, C1-6alkoxy, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, and C1- 6alkoxyC1-6alkyl, wherein each of said groups is optionally substituted with one to three substituents independently selected from halogen and CN. In a particular embodiment, there is provided a compound of formula (I) according to the present invention, wherein
Z is O; A is N; the four A
1 are CR
1 with R
1 being independently selected from hydrogen, hydroxy, halogen, CN, C1- 6alkyl, C3-6cycloalkyl, C1-6alkoxy-C1-6alkyl, C3-6cycloalkyl-C1-4alkyl, C1-6alkylsulfanyl, C1-6alkylsulfinyl, C1- 6alkylsulfonyl and C1-6alkoxy; and preferably
, the four A
2 are CR
2; with R
2 being independently selected from hydrogen, hydroxy, halogen, CN, C1- 6alkyl, C1-6alkoxy, C1-6alkoxy-C1-6alkyl, C1-6alkoxy-C1-6alkoxy, C2-6alkenyl, C2-6alkynyl, C3-6cycloalkyl, C3-
6cycloalkyl-C
1-6alkyl, C
1-6alkylsulfanyl, C
1-6alkylsulfinyl, and C
1-6alkylsulfonyl, wherein each of the C
1- 6alkyl, C1-6alkoxy, C1-6alkoxy-C1-6alkyl, C1-6alkoxy-C1-6alkoxy, C2-6alkenyl, C2-6alkynyl, C3-6cycloalkyl, C3- 6cycloalkyl-C1-6alkyl, C1-6alkylsulfanyl, C1-6alkylsulfinyl and C1-6alkylsulfonyl groups is optionally substituted with one to three substituents independently selected from halogen, hydroxy, and CN; and preferably R
2 being independently selected from hydrogen, halogen, CN, C1-6alkyl, C1-6alkoxy, C1- 6alkoxy-C1-6alkyl, and C1-6alkoxy-C1-6alkoxy, wherein each of the C1-6alkyl, C1-6alkoxy, C1-6alkoxy-C1- 6alkyl, and C1-6alkoxy-C1-6alkoxy groups is optionally substituted with one to three substituents independently selected from halogen, hydroxy, and CN; A
3 is CR
3 with R
3 being independently selected from hydrogen, hydroxy, halogen, CN, C
1-6alkyl, C
1- 6alkoxy, C1-6alkoxy-C1-6alkyl, C1-6alkoxy-C1-6alkoxy, C2-6alkenyl, C2-6alkynyl, C3-6cycloalkyl, C3- 6cycloalkyl-C1-6alkyl, C1-6alkylsulfanyl, C1-6alkylsulfinyl, C1-6alkylsulfonyl, amino, C1-6alkylamino, diC1-6- alkylamino, and C3-6cycloalkylamino, wherein each of the C1-6alkyl, C1-6alkoxy, C1-6alkoxy-C1-6alkyl, C1- 6alkoxy-C1-6alkoxy, C2-6alkenyl, C2-6alkynyl, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, C1-6alkylsulfanyl, C1- 6alkylsulfinyl, C1-6alkylsulfonyl, amino, C1-6alkylamino, diC1-6-alkylamino and C3-6cycloalkylamino groups is optionally substituted with one to three substituents independently selected from halogen, hydroxy, and CN; and preferably R
3 being hydrogen; R
4 is selected from C
1-6alkyl, C
1-6alkoxy-C
1-6alkyl, C
3-6cycloalkyl, C
3-6cycloalkyl-C
1-4alkyl, and C
1-6alkoxy, wherein each of the C1-6alkyl, C1-6alkoxy-C1-6alkyl, C3-6cycloalkyl, C3-6cycloalkyl-C1-4alkyl, and C1-6alkoxy groups is optionally substituted with one to three substituents independently selected from halogen and CN; and wherein A
3 and R
4 taken together optionally form a ring, more preferably a 5-8-membered heterocycle, more preferably a 6-membered heterocycle; and R
5 is selected from C1-6alkyl, C1-6alkoxy, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, C1-6alkoxyC1-6 alkyl, C1- 6alkylamino, diC1-6alkylamino, and C1-6alkylC1-6alkoxyamino, wherein each of said groups is optionally substituted with one to three substituents independently selected from halogen and CN, and preferably
R
5 is selected from C1-6alkyl, C1-6alkoxy, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, and C1-6alkoxyC1-6alkyl, wherein each of said groups is optionally substituted with one to three substituents independently selected from halogen and CN. In a preferred embodiment,
, wherein R
2 is as defined in the present invention; preferably R
2 are independently selected from hydrogen, hydroxy, halogen, CN, C1-6alkyl, C1-6alkoxy, C1-6alkoxy-C1-6alkyl, C1-6alkoxy-C1-6alkoxy, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, C1-6alkoxycarbonyl, C1-6alkylaminocarbonyl, diC1-6alkylaminocarbonyl, and C1-6alkylcarbonyl, wherein each of the C1-6alkyl, C1-6alkoxy, C1-6alkoxy-C1-6alkyl, C1-6alkoxy-C1-6alkoxy, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, C1- 6alkoxycarbonyl, C1-6alkylaminocarbonyl, diC1-6alkylaminocarbonyl, and C1-6alkylcarbonyl groups is optionally substituted with one to three substituents independently selected from halogen, hydroxy, and CN; preferably R
2 are independently selected from hydrogen, halogen, CN, C1-6alkoxy, C1-6alkoxy-C1- 6alkyl, and C1-6alkoxy-C1-6alkoxy, wherein each of the C1-6alkoxy, C1-6alkoxy-C1-6alkyl, and C1-6alkoxy- C1-6alkoxy groups is optionally substituted with one to three substituents independently selected from halogen, hydroxy, and CN, and more preferably R
2 are independently selected from hydrogen, halogen, CN, and C1-6alkoxy, wherein the C1-6alkoxy group is optionally substituted with one to three substituents independently selected from halogen, hydroxy, and CN. In a preferred embodiment,
, wherein R
2 is as defined in the present invention; preferably R
2 is selected from hydroxy, halogen, CN, C1-6alkyl, C1-6alkoxy, C1-6alkoxy-C1-6alkyl, C1- 6alkoxy-C1-6alkoxy, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, C1-6alkoxycarbonyl, C1-6alkylaminocarbonyl, diC1-6alkylaminocarbonyl, and C1-6alkylcarbonyl, wherein each of the C1-6alkyl, C1-6alkoxy, C1-6alkoxy- C1-6alkyl, C1-6alkoxy-C1-6alkoxy, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, C1-6alkoxycarbonyl, C1- 6alkylaminocarbonyl, diC1-6alkylaminocarbonyl, and C1-6alkylcarbonyl groups is optionally substituted with one to three substituents independently selected from halogen, hydroxy, and CN; and more preferably R
2 are independently selected from halogen, CN, C1-6alkoxy, and C1-6alkoxy-C1-6alkyl, wherein each of the C1-6alkoxy and C1-6alkoxy-C1-6alkyl groups is optionally substituted with one to three substituents independently selected from halogen, hydroxy, and CN. In a further embodiment, the compound according to the present invention is selected from: methyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]-4-(methoxymethyl)benzimidazol-1-yl]- 2-pyridyl]carbamate; methyl N-[5-[6-[2-cyanoethyl-(4-fluoro-3-methoxy-phenyl)carbamoyl]-4-methyl-benzimidazol-1-yl]-2- pyridyl]carbamate; methyl N-[5-[4-ethyl-6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]benzimidazol-1-yl]-2- pyridyl]carbamate; methyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-(methoxymethyl)carbamoyl]-4- (methoxymethyl)benzimidazol-1-yl]-2-pyridyl]carbamate;
methyl N-[5-[6-[ethyl-(4-fluoro-3-methoxy-phenyl)carbamoyl]-4-(methoxymethyl)benzimidazol-1-yl]-2- pyridyl]carbamate; methyl N-[5-[4-ethyl-6-[(4-fluoro-3-methoxy-phenyl)-(methoxymethyl)carbamoyl]benzimidazol-1-yl]-2- pyridyl]carbamate; methyl N-[5-[6-[cyanomethyl-(4-fluorophenyl)carbamoyl]-4-methyl-benzimidazol-1-yl]-2- pyridyl]carbamate; methyl N-[5-[6-[cyanomethyl-(4-fluoro-3-methoxy-phenyl)carbamoyl]-4-methyl-benzimidazol-1-yl]-2- pyridyl]carbamate; methyl N-[5-[6-[(4-cyano-3-methoxy-phenyl)-methyl-carbamoyl]-4-methyl-benzimidazol-1-yl]-2- pyridyl]carbamate; methyl N-[5-[6-[(4-cyano-3-fluoro-phenyl)-methyl-carbamoyl]-4-methyl-benzimidazol-1-yl]-2- pyridyl]carbamate; methyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]-4-methyl-imidazo[4,5-c]pyridin-1-yl]-2- pyridyl]carbamate; methyl N-[5-[5-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]imidazo[4,5-b]pyridin-3-yl]-2- pyridyl]carbamate; methyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-(2-methoxyethyl)carbamoyl]-4-methyl-benzimidazol-1-yl]- 2-pyridyl]carbamate; 3-(6-acetamido-3-pyridyl)-N-(4-fluoro-3-methoxy-phenyl)-N,7-dimethyl-benzimidazole-5-carboxamide; N-(4-fluoro-3-methoxy-phenyl)-N,7-dimethyl-3-[6-(methylcarbamoylamino)-3-pyridyl]benzimidazole-5- carboxamide; ethyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]-4-methyl-benzimidazol-1-yl]-2- pyridyl]carbamate; methyl N-[5-[4-ethyl-6-[(4-fluorophenyl)-(methoxymethyl)carbamoyl]benzimidazol-1-yl]-2- pyridyl]carbamate; 3-[6-(cyclopropanecarbonylamino)-3-pyridyl]-N-(4-fluoro-3-methoxy-phenyl)-N,7-dimethyl- benzimidazole-5-carboxamide; methyl N-[5-[6-[(2-methoxy-4-pyridyl)-methyl-carbamoyl]-4-methyl-benzimidazol-1-yl]-2- pyridyl]carbamate; methyl N-[5-[6-(6-fluoro-4-methyl-2,3-dihydroquinoxaline-1-carbonyl)-4-methyl-benzimidazol-1-yl]-2- pyridyl]carbamate; methyl N-[5-[5-[(4-fluorophenyl)-methyl-carbamoyl]-7-methyl-imidazo[4,5-b]pyridin-3-yl]-2- pyridyl]carbamate; methyl N-[5-[6-[(4-fluorophenyl)-methyl-carbamoyl]benzimidazol-1-yl]-2-pyridyl]carbamate; methyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-(methoxymethyl)carbamoyl]-4-methyl-benzimidazol-1-yl]- 2-pyridyl]carbamate; methyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]imidazo[4,5-c]pyridin-1-yl]-2- pyridyl]carbamate; methyl N-[5-[6-[(4-fluorophenyl)-methyl-carbamoyl]-4-methyl-benzimidazol-1-yl]-2-pyridyl]carbamate; methyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]-4-methyl-benzimidazol-1-yl]-2- pyridyl]carbamate;
methyl N-[5-[6-[(4-fluorophenyl)-(methoxymethyl)carbamoyl]-4-(methoxymethyl)benzimidazol-1-yl]-2- pyridyl]carbamate; methyl N-[5-[6-[(4-fluorophenyl)-(2-methoxyethyl)carbamoyl]-4-methyl-benzimidazol-1-yl]-2- pyridyl]carbamate; methyl N-[5-[6-[2-cyanoethyl-(4-fluorophenyl)carbamoyl]-4-methyl-benzimidazol-1-yl]-2- pyridyl]carbamate; 3-(6-acetamido-3-pyridyl)-N-(4-fluorophenyl)-N,7-dimethyl-benzimidazole-5-carboxamide; methyl N-[5-[6-[(4-fluorophenyl)-methyl-carbamoyl]-4-(methoxymethyl)benzimidazol-1-yl]-2- pyridyl]carbamate; methyl N-[5-[2-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]-6-methyl-purin-9-yl]-2- pyridyl]carbamate; methyl N-[5-[6-(6-fluoro-3,4-dihydro-2H-quinoline-1-carbonyl)-4-methyl-benzimidazol-1-yl]-2- pyridyl]carbamate; methyl N-[5-[6-[ethyl-(4-fluorophenyl)carbamoyl]-4-(methoxymethyl)benzimidazol-1-yl]-2- pyridyl]carbamate; methyl N-[5-[5-(6-fluoro-3,4-dihydro-2H-quinoline-1-carbonyl)-7-methyl-imidazo[4,5-b]pyridin-3-yl]-2- pyridyl]carbamate; methyl N-[5-[5-[(4-fluoro-3-methyl-phenyl)-methyl-carbamoyl]-7-methyl-imidazo[4,5-b]pyridin-3-yl]-2- pyridyl]carbamate; methyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]imidazo[4,5-b]pyridin-1-yl]-2- pyridyl]carbamate; methyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]benzimidazol-1-yl]-2-pyridyl]carbamate; methyl N-[5-[6-[(4-fluorophenyl)-methyl-carbamoyl]-4-methyl-imidazo[4,5-c]pyridin-1-yl]-2- pyridyl]carbamate; methyl N-[5-[5-[(4-fluoro-3-methyl-phenyl)-methyl-carbamoyl]imidazo[4,5-b]pyridin-3-yl]-2- pyridyl]carbamate; methyl N-[5-[6-[(4-fluorophenyl)-methyl-carbamoyl]imidazo[4,5-b]pyridin-1-yl]-2-pyridyl]carbamate; methyl N-[5-[6-[(4-fluorophenyl)-methyl-carbamoyl]imidazo[4,5-c]pyridin-1-yl]-2-pyridyl]carbamate; methyl N-[5-[2-[(4-fluorophenyl)-methyl-carbamoyl]purin-9-yl]-2-pyridyl]carbamate; 3-[6-(cyclopropanecarbonylamino)-3-pyridyl]-N-(4-fluorophenyl)-N,7-dimethyl-benzimidazole-5- carboxamide; N-(4-fluorophenyl)-N,7-dimethyl-3-[6-(methylcarbamoylamino)-3-pyridyl]benzimidazole-5-carboxamide; N-(4-fluorophenyl)-N,7-dimethyl-3-[6-(1H-pyrazole-5-carbonylamino)-3-pyridyl]benzimidazole-5- carboxamide; N-(4-fluoro-3-methoxy-phenyl)-N,7-dimethyl-3-[6-(1H-pyrazole-5-carbonylamino)-3- pyridyl]benzimidazole-5-carboxamide; N-(4-fluoro-3-methoxy-phenyl)-N,7-dimethyl-3-[6-(1H-pyrazole-4-carbonylamino)-3- pyridyl]benzimidazole-5-carboxamide; N-(4-fluorophenyl)-N,7-dimethyl-3-[6-(1H-pyrazole-4-carbonylamino)-3-pyridyl]benzimidazole-5- carboxamide; N-(4-fluorophenyl)-N,7-dimethyl-3-[6-[(1-methylpyrazole-4-carbonyl)amino]-3-pyridyl]benzimidazole-5- carboxamide;
N-(4-fluoro-3-methoxy-phenyl)-N,7-dimethyl-3-[6-[(1-methylpyrazole-4-carbonyl)amino]-3- pyridyl]benzimidazole-5-carboxamide; N-(4-fluoro-3-methoxy-phenyl)-N,7-dimethyl-3-[6-[(2-methylpyrazole-3-carbonyl)amino]-3- pyridyl]benzimidazole-5-carboxamide; N-(4-fluorophenyl)-N,7-dimethyl-3-[6-[(2-methylpyrazole-3-carbonyl)amino]-3-pyridyl]benzimidazole-5- carboxamide; ethyl N-[5-[6-[(4-fluorophenyl)-methyl-carbamoyl]-4-methyl-benzimidazol-1-yl]-2-pyridyl]carbamate; methyl N-[5-[4-ethyl-6-[(4-fluorophenyl)-methyl-carbamoyl]benzimidazol-1-yl]-2-pyridyl]carbamate; methyl N-[5-[2-[(4-fluorophenyl)-methyl-carbamoyl]-6-methyl-purin-9-yl]-2-pyridyl]carbamate; methyl N-[5-[5-[(4-fluorophenyl)-methyl-carbamoyl]imidazo[4,5-b]pyrazin-3-yl]-2-pyridyl]carbamate; methyl N-[5-[2-[(4-fluoro-3-methyl-phenyl)-methyl-carbamoyl]purin-9-yl]-2-pyridyl]carbamate; methyl N-[5-[2-(6-fluoro-3,4-dihydro-2H-quinoline-1-carbonyl)purin-9-yl]-2-pyridyl]carbamate; methyl N-[5-[5-(6-fluoro-3,4-dihydro-2H-quinoline-1-carbonyl)imidazo[4,5-b]pyridin-3-yl]-2- pyridyl]carbamate; methyl N-[5-[6-(6-fluoro-3,4-dihydro-2H-quinoline-1-carbonyl)imidazo[4,5-b]pyridin-1-yl]-2- pyridyl]carbamate; methyl N-[5-[5-[(4-fluorophenyl)-methyl-carbamoyl]imidazo[4,5-b]pyridin-3-yl]-2-pyridyl]carbamate; methyl N-[5-[6-[(4-fluoro-3-methyl-phenyl)-methyl-carbamoyl]imidazo[4,5-b]pyridin-1-yl]-2- pyridyl]carbamate; methyl N-[5-[6-(7-fluoro-2,3-dihydro-1,4-benzoxazine-4-carbonyl)-4-methyl-benzimidazol-1-yl]-2- pyridyl]carbamate; methyl N-[5-[2-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]purin-9-yl]-2-pyridyl]carbamate; methyl N-[4-[2-[(4-chlorophenyl)-methyl-carbamoyl]purin-9-yl]phenyl]carbamate; 9-(4-acetamidophenyl)-N-(4-chlorophenyl)-N-methyl-purine-2-carboxamide; 3-(4-acetamidophenyl)-N-(4-chlorophenyl)-N-methyl-benzotriazole-5-carboxamide; methyl N-[4-[6-[(4-chlorophenyl)-methyl-carbamoyl]benzotriazol-1-yl]phenyl]carbamate; 1-(4-acetamidophenyl)-N-(4-chlorophenyl)-N-methyl-imidazo[4,5-c]pyridine-6-carboxamide; N-(4-chlorophenyl)-3-[6-[(2-methoxyacetyl)amino]-3-pyridyl]-N,7-dimethyl-benzimidazole-5- carboxamide; N-(4-fluoro-3-methoxy-phenyl)-3-[6-[(2-methoxyacetyl)amino]-3-pyridyl]-N,7-dimethyl-benzimidazole-5- carboxamide N-(3,4-difluorophenyl)-3-[6-[(2-methoxyacetyl)amino]-3-pyridyl]-N,7-dimethyl-benzimidazole-5- carboxamide; 3-[4-(cyclopropanecarbonylamino)phenyl]-N-(3,4-difluorophenyl)-N,7-dimethyl-benzimidazole-5- carboxamide; 3-(6-acetamido-3-pyridyl)-N-(4-fluoro-3-methyl-phenyl)-N,7-dimethyl-benzimidazole-5-carboxamide; 3-[6-(cyclopropanecarbonylamino)-3-pyridyl]-N-(4-fluoro-3-methyl-phenyl)-N,7-dimethyl- benzimidazole-5-carboxamide; tert-butyl N-[4-[6-[(3,4-difluorophenyl)-methyl-carbamoyl]benzimidazol-1-yl]phenyl]carbamate; 3-[6-(cyclopropanecarbonylamino)-3-pyridyl]-N-(4-fluoro-3-methoxy-phenyl)-N-methyl-benzimidazole- 5-carboxamide;
methyl N-[4-[6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]-4-mCSethyl-benzimidazol-1- yl]phenyl]carbamate; tert-butyl N-[4-[6-[(3,4-difluorophenyl)-methyl-carbamoyl]-4-methyl-benzimidazol-1- yl]phenyl]carbamate; benzyl N-[4-[6-[(3,4-difluorophenyl)-methyl-carbamoyl]-4-methyl-benzimidazol-1-yl]phenyl]carbamate; methyl N-[4-[6-[(3,4-difluorophenyl)-methyl-carbamoyl]-4-methyl-benzimidazol-1-yl]phenyl]carbamate; 3-(4-acetamidophenyl)-N-(3,4-difluorophenyl)-N,7-dimethyl-benzimidazole-5-carboxamide; benzyl N-[4-[6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]-4-methyl-benzimidazol-1- yl]phenyl]carbamate; tert-butyl N-[4-[6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]-4-methyl-benzimidazol-1- yl]phenyl]carbamate; N-(4-cyanophenyl)-3-[6-(cyclopropanecarbonylamino)-3-pyridyl]-N,7-dimethyl-benzimidazole-5- carboxamide; N-(4-chlorophenyl)-3-[6-(cyclopropanecarbonylamino)-3-pyridyl]-N,7-dimethyl-benzimidazole-5- carboxamide; N-(4-fluoro-3-methoxy-phenyl)-3-(6-formamido-3-pyridyl)-N-methyl-benzimidazole-5-carboxamide; N-(4-cyano-3-methoxy-phenyl)-3-[6-(cyclopropanecarbonylamino)-3-pyridyl]-N,7-dimethyl- benzimidazole-5-carboxamide; N-(4-fluoro-3-methoxy-phenyl)-3-[6-[(2-methoxyacetyl)amino]-3-pyridyl]-N-methyl-benzimidazole-5- carboxamide; methyl N-[5-[6-[(4-cyanophenyl)-methyl-carbamoyl]-4-methyl-benzimidazol-1-yl]-2-pyridyl]carbamate; 3-(6-acetamido-3-pyridyl)-N-(3,4-difluorophenyl)-N,7-dimethyl-benzimidazole-5-carboxamide; 3-(4-acetamidophenyl)-N-(4-fluoro-3-methoxy-phenyl)-N-methyl-benzimidazole-5-carboxamide; 3-(6-acetamido-3-pyridyl)-N-(4-fluoro-3-methoxy-phenyl)-N-(methoxymethyl)-7-methyl-benzimidazole- 5-carboxamide; methyl N-[5-[6-[(4-chlorophenyl)-methyl-carbamoyl]-4-methyl-benzimidazol-1-yl]-2-pyridyl]carbamate; methyl N-[5-[6-[(3,4-difluorophenyl)-methyl-carbamoyl]-4-methyl-benzimidazol-1-yl]-2- pyridyl]carbamate; 3-[6-(cyclopropanecarbonylamino)-3-pyridyl]-N-(4-fluoro-3-methoxy-phenyl)-N-(methoxymethyl)-7- methyl-benzimidazole-5-carboxamide; and methyl N-[5-[6-[(4-chlorophenyl)-methyl-carbamothioyl]-4-methyl-benzimidazol-1-yl]-2- pyridyl]carbamate. The method according to the present invention has advantageous properties for protecting plants against pathogenic, such as phytopathogenic, especially fungi such as oomycetes, attack or infestation, which result in a disease and damage to the plant; particularly in instance of plants, the present invention can control, limit or prevent pathogenic damage on plant, parts of plant, plant propagation material and/or plant grown. The compounds in Tables 1.1 to 1.132 below illustrate the compounds of the invention. Table 1.1 provides 700 compounds E1.1 to E1.700 of formula (Ia)
wherein A is CH, R
2 is H, R
5 is CH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table Z: Substituent definitions of A
2a, A
2b, R
1, R
4 and A
6 Compounds A
2b R
1 R
4 A
6 A
2a E1.1 CH H CH3 CH CH E1.2 CH H CH3 N CH E1.3 CH H CH2CH3 CH CH E1.4 CH H CH2CH3 N CH E1.5 CH H CH2OCH3 CH CH E1.6 CH H CH2OCH3 N CH E1.7 CH H CH2CH2OCH3 CH CH E1.8 CH H CH2CH2OCH3 N CH E1.9 CH H CH2CN CH CH E1.10 CH H CH2CN N CH E1.11 CH CH3 CH3 CH CH E1.12 CH CH3 CH3 N CH E1.13 CH CH3 CH2CH3 CH CH E1.14 CH CH3 CH2CH3 N CH E1.15 CH CH3 CH2OCH3 CH CH E1.16 CH CH3 CH2OCH3 N CH E1.17 CH CH3 CH2CH2OCH3 CH CH E1.18 CH CH3 CH2CH2OCH3 N CH E1.19 CH CH3 CH2CN CH CH E1.20 CH CH3 CH2CN N CH E1.21 CH CH2OCH3 CH3 CH CH E1.22 CH CH2OCH3 CH3 N CH E1.23 CH CH2OCH3 CH2CH3 CH CH E1.24 CH CH2OCH3 CH2CH3 N CH E1.25 CH CH2OCH3 CH2OCH3 CH CH E1.26 CH CH2OCH3 CH2OCH3 N CH E1.27 CH CH2OCH3 CH2CH2OCH3 CH CH E1.28 CH CH2OCH3 CH2CH2OCH3 N CH
Compounds A
2b R
1 R
4 A
6 A
2a E1.29 CH CH2OCH3 CH2CN CH CH E1.30 CH CH2OCH3 CH2CN N CH E1.31 CH CH3 CH CH E1.32 CH CH3 N CH E1.33 CH CH2CH3 CH CH E1.34 CH CH2CH3 N CH E1.35 CH CH2OCH3 CH CH E1.36 CH CH2OCH3 N CH E1.37 CH CH2CH2OCH3 CH CH E1.38 CH CH2CH2OCH3 N CH E1.39 CH CH2CN CH CH E1.40 CH CH2CN N CH E1.41 CH NHAc CH3 CH CH E1.42 CH NHAc CH3 N CH E1.43 CH NHAc CH2CH3 CH CH E1.44 CH NHAc CH2CH3 N CH E1.45 CH NHAc CH2OCH3 CH CH E1.46 CH NHAc CH2OCH3 N CH E1.47 CH NHAc CH2CH2OCH3 CH CH E1.48 CH NHAc CH2CH2OCH3 N CH E1.49 CH NHAc CH2CN CH CH E1.50 CH NHAc CH2CN N CH E1.51 N H CH3 CH CH E1.52 N H CH3 N CH E1.53 N H CH2CH3 CH CH E1.54 N H CH2CH3 N CH E1.55 N H CH2OCH3 CH CH E1.56 N H CH2OCH3 N CH E1.57 N H CH2CH2OCH3 CH CH E1.58 N H CH2CH2OCH3 N CH E1.59 N H CH2CN CH CH E1.60 N H CH2CN N CH E1.61 N CH3 CH3 CH CH E1.62 N CH3 CH3 N CH E1.63 N CH3 CH2CH3 CH CH E1.64 N CH3 CH2CH3 N CH
Compounds A
2b R
1 R
4 A
6 A
2a E1.65 N CH3 CH2OCH3 CH CH E1.66 N CH3 CH2OCH3 N CH E1.67 N CH3 CH2CH2OCH3 CH CH E1.68 N CH3 CH2CH2OCH3 N CH E1.69 N CH3 CH2CN CH CH E1.70 N CH3 CH2CN N CH E1.71 N CH2OCH3 CH3 CH CH E1.72 N CH2OCH3 CH3 N CH E1.73 N CH2OCH3 CH2CH3 CH CH E1.74 N CH2OCH3 CH2CH3 N CH E1.75 N CH2OCH3 CH2OCH3 CH CH E1.76 N CH2OCH3 CH2OCH3 N CH E1.77 N CH2OCH3 CH2CH2OCH3 CH CH E1.78 N CH2OCH3 CH2CH2OCH3 N CH E1.79 N CH2OCH3 CH2CN CH CH E1.80 N CH2OCH3 CH2CN N CH E1.81 N CH3 CH CH E1.82 N CH3 N CH E1.83 N CH2CH3 CH CH E1.84 N CH2CH3 N CH E1.85 N CH2OCH3 CH CH E1.86 N CH2OCH3 N CH E1.87 N CH2CH2OCH3 CH CH E1.88 N CH2CH2OCH3 N CH E1.89 N CH2CN CH CH E1.90 N CH2CN N CH E1.91 N NHAc CH3 CH CH E1.92 N NHAc CH3 N CH E1.93 N NHAc CH2CH3 CH CH E1.94 N NHAc CH2CH3 N CH E1.95 N NHAc CH2OCH3 CH CH E1.96 N NHAc CH2OCH3 N CH E1.97 N NHAc CH2CH2OCH3 CH CH E1.98 N NHAc CH2CH2OCH3 N CH E1.99 N NHAc CH2CN CH CH E1.100 N NHAc CH2CN N CH
Compounds A
2b R
1 R
4 A
6 A
2a E1.101 CH H CH3 CH N E1.102 CH H CH3 N N E1.103 CH H CH2CH3 CH N E1.104 CH H CH2CH3 N N E1.105 CH H CH2OCH3 CH N E1.106 CH H CH2OCH3 N N E1.107 CH H CH2CH2OCH3 CH N E1.108 CH H CH2CH2OCH3 N N E1.109 CH H CH2CN CH N E1.110 CH H CH2CN N N E1.111 CH CH3 CH3 CH N E1.112 CH CH3 CH3 N N E1.113 CH CH3 CH2CH3 CH N E1.114 CH CH3 CH2CH3 N N E1.115 CH CH3 CH2OCH3 CH N E1.116 CH CH3 CH2OCH3 N N E1.117 CH CH3 CH2CH2OCH3 CH N E1.118 CH CH3 CH2CH2OCH3 N N E1.119 CH CH3 CH2CN CH N E1.120 CH CH3 CH2CN N N E1.121 CH CH2OCH3 CH3 CH N E1.122 CH CH2OCH3 CH3 N N E1.123 CH CH2OCH3 CH2CH3 CH N E1.124 CH CH2OCH3 CH2CH3 N N E1.125 CH CH2OCH3 CH2OCH3 CH N E1.126 CH CH2OCH3 CH2OCH3 N N E1.127 CH CH2OCH3 CH2CH2OCH3 CH N E1.128 CH CH2OCH3 CH2CH2OCH3 N N E1.129 CH CH2OCH3 CH2CN CH N E1.130 CH CH2OCH3 CH2CN N N E1.131 CH CH3 CH N E1.132 CH CH3 N N E1.133 CH CH2CH3 CH N E1.134 CH CH2CH3 N N E1.135 CH CH2OCH3 CH N E1.136 CH CH2OCH3 N N E1.137 CH CH2CH2OCH3 CH N
Compounds A
2b R
1 R
4 A
6 A
2a E1.138 CH CH2CH2OCH3 N N E1.139 CH CH2CN CH N E1.140 CH CH2CN N N E1.141 CH NHAc CH3 CH N E1.142 CH NHAc CH3 N N E1.143 CH NHAc CH2CH3 CH N E1.144 CH NHAc CH2CH3 N N E1.145 CH NHAc CH2OCH3 CH N E1.146 CH NHAc CH2OCH3 N N E1.147 CH NHAc CH2CH2OCH3 CH N E1.148 CH NHAc CH2CH2OCH3 N N E1.149 CH NHAc CH2CN CH N E1.150 CH NHAc CH2CN N N E1.151 N H CH3 CH N E1.152 N H CH3 N N E1.153 N H CH2CH3 CH N E1.154 N H CH2CH3 N N E1.155 N H CH2OCH3 CH N E1.156 N H CH2OCH3 N N E1.157 N H CH2CH2OCH3 CH N E1.158 N H CH2CH2OCH3 N N E1.159 N H CH2CN CH N E1.160 N H CH2CN N N E1.161 N CH3 CH3 CH N E1.162 N CH3 CH3 N N E1.163 N CH3 CH2CH3 CH N E1.164 N CH3 CH2CH3 N N E1.165 N CH3 CH2OCH3 CH N E1.166 N CH3 CH2OCH3 N N E1.167 N CH3 CH2CH2OCH3 CH N E1.168 N CH3 CH2CH2OCH3 N N E1.169 N CH3 CH2CN CH N E1.170 N CH3 CH2CN N N E1.171 N CH2OCH3 CH3 CH N E1.172 N CH2OCH3 CH3 N N E1.173 N CH2OCH3 CH2CH3 CH N E1.174 N CH2OCH3 CH2CH3 N N E1.175 N CH2OCH3 CH2OCH3 CH N E1.176 N CH2OCH3 CH2OCH3 N N
Compounds A
2b R
1 R
4 A
6 A
2a E1.177 N CH2OCH3 CH2CH2OCH3 CH N E1.178 N CH2OCH3 CH2CH2OCH3 N N E1.179 N CH2OCH3 CH2CN CH N E1.180 N CH2OCH3 CH2CN N N E1.181 N CH3 CH N E1.182 N CH3 N N E1.183 N CH2CH3 CH N E1.184 N CH2CH3 N N E1.185 N CH2OCH3 CH N E1.186 N CH2OCH3 N N E1.187 N CH2CH2OCH3 CH N E1.188 N CH2CH2OCH3 N N E1.189 N CH2CN CH N E1.190 N CH2CN N N E1.191 N NHAc CH3 CH N E1.192 N NHAc CH3 N N E1.193 N NHAc CH2CH3 CH N E1.194 N NHAc CH2CH3 N N E1.195 N NHAc CH2OCH3 CH N E1.196 N NHAc CH2OCH3 N N E1.197 N NHAc CH2CH2OCH3 CH N E1.198 N NHAc CH2CH2OCH3 N N E1.199 N NHAc CH2CN CH N E1.200 N NHAc CH2CN N N E1.201 CH H CH3 CH CF E1.202 CH H CH3 N CF E1.203 CH H CH2CH3 CH CF E1.204 CH H CH2CH3 N CF E1.205 CH H CH2OCH3 CH CF E1.206 CH H CH2OCH3 N CF E1.207 CH H CH2CH2OCH3 CH CF E1.208 CH H CH2CH2OCH3 N CF E1.209 CH H CH2CN CH CF E1.210 CH H CH2CN N CF E1.211 CH CH3 CH3 CH CF E1.212 CH CH3 CH3 N CF
Compounds A
2b R
1 R
4 A
6 A
2a E1.213 CH CH3 CH2CH3 CH CF E1.214 CH CH3 CH2CH3 N CF E1.215 CH CH3 CH2OCH3 CH CF E1.216 CH CH3 CH2OCH3 N CF E1.217 CH CH3 CH2CH2OCH3 CH CF E1.218 CH CH3 CH2CH2OCH3 N CF E1.219 CH CH3 CH2CN CH CF E1.220 CH CH3 CH2CN N CF E1.221 CH CH2OCH3 CH3 CH CF E1.222 CH CH2OCH3 CH3 N CF E1.223 CH CH2OCH3 CH2CH3 CH CF E1.224 CH CH2OCH3 CH2CH3 N CF E1.225 CH CH2OCH3 CH2OCH3 CH CF E1.226 CH CH2OCH3 CH2OCH3 N CF E1.227 CH CH2OCH3 CH2CH2OCH3 CH CF E1.228 CH CH2OCH3 CH2CH2OCH3 N CF E1.229 CH CH2OCH3 CH2CN CH CF E1.230 CH CH2OCH3 CH2CN N CF E1.231 CH CH3 CH CF E1.232 CH CH3 N CF E1.233 CH CH2CH3 CH CF E1.234 CH CH2CH3 N CF E1.235 CH CH2OCH3 CH CF E1.236 CH CH2OCH3 N CF E1.237 CH CH2CH2OCH3 CH CF E1.238 CH CH2CH2OCH3 N CF E1.239 CH CH2CN CH CF E1.240 CH CH2CN N CF E1.241 CH NHAc CH3 CH CF E1.242 CH NHAc CH3 N CF E1.243 CH NHAc CH2CH3 CH CF E1.244 CH NHAc CH2CH3 N CF E1.245 CH NHAc CH2OCH3 CH CF E1.246 CH NHAc CH2OCH3 N CF E1.247 CH NHAc CH2CH2OCH3 CH CF E1.248 CH NHAc CH2CH2OCH3 N CF
Compounds A
2b R
1 R
4 A
6 A
2a E1.249 CH NHAc CH2CN CH CF E1.250 CH NHAc CH2CN N CF E1.251 N H CH3 CH CF E1.252 N H CH3 N CF E1.253 N H CH2CH3 CH CF E1.254 N H CH2CH3 N CF E1.255 N H CH2OCH3 CH CF E1.256 N H CH2OCH3 N CF E1.257 N H CH2CH2OCH3 CH CF E1.258 N H CH2CH2OCH3 N CF E1.259 N H CH2CN CH CF E1.260 N H CH2CN N CF E1.261 N CH3 CH3 CH CF E1.262 N CH3 CH3 N CF E1.263 N CH3 CH2CH3 CH CF E1.264 N CH3 CH2CH3 N CF E1.265 N CH3 CH2OCH3 CH CF E1.266 N CH3 CH2OCH3 N CF E1.267 N CH3 CH2CH2OCH3 CH CF E1.268 N CH3 CH2CH2OCH3 N CF E1.269 N CH3 CH2CN CH CF E1.270 N CH3 CH2CN N CF E1.271 N CH2OCH3 CH3 CH CF E1.272 N CH2OCH3 CH3 N CF E1.273 N CH2OCH3 CH2CH3 CH CF E1.274 N CH2OCH3 CH2CH3 N CF E1.275 N CH2OCH3 CH2OCH3 CH CF E1.276 N CH2OCH3 CH2OCH3 N CF E1.277 N CH2OCH3 CH2CH2OCH3 CH CF E1.278 N CH2OCH3 CH2CH2OCH3 N CF E1.279 N CH2OCH3 CH2CN CH CF E1.280 N CH2OCH3 CH2CN N CF E1.281 N CH3 CH CF E1.282 N CH3 N CF E1.283 N CH2CH3 CH CF E1.284 N CH2CH3 N CF E1.285 N CH2OCH3 CH CF E1.286 N CH2OCH3 N CF
Compounds A
2b R
1 R
4 A
6 A
2a E1.287 N CH2CH2OCH3 CH CF E1.288 N CH2CH2OCH3 N CF E1.289 N CH2CN CH CF E1.290 N CH2CN N CF E1.291 N NHAc CH3 CH CF E1.292 N NHAc CH3 N CF E1.293 N NHAc CH2CH3 CH CF E1.294 N NHAc CH2CH3 N CF E1.295 N NHAc CH2OCH3 CH CF E1.296 N NHAc CH2OCH3 N CF E1.297 N NHAc CH2CH2OCH3 CH CF E1.298 N NHAc CH2CH2OCH3 N CF E1.299 N NHAc CH2CN CH CF E1.300 N NHAc CH2CN N CF E1.301 CH H CH3 CH CCl E1.302 CH H CH3 N CCl E1.303 CH H CH2CH3 CH CCl E1.304 CH H CH2CH3 N CCl E1.305 CH H CH2OCH3 CH CCl E1.306 CH H CH2OCH3 N CCl E1.307 CH H CH2CH2OCH3 CH CCl E1.308 CH H CH2CH2OCH3 N CCl E1.309 CH H CH2CN CH CCl E1.310 CH H CH2CN N CCl E1.311 CH CH3 CH3 CH CCl E1.312 CH CH3 CH3 N CCl E1.313 CH CH3 CH2CH3 CH CCl E1.314 CH CH3 CH2CH3 N CCl E1.315 CH CH3 CH2OCH3 CH CCl E1.316 CH CH3 CH2OCH3 N CCl E1.317 CH CH3 CH2CH2OCH3 CH CCl E1.318 CH CH3 CH2CH2OCH3 N CCl E1.319 CH CH3 CH2CN CH CCl E1.320 CH CH3 CH2CN N CCl E1.321 CH CH2OCH3 CH3 CH CCl E1.322 CH CH2OCH3 CH3 N CCl E1.323 CH CH2OCH3 CH2CH3 CH CCl E1.324 CH CH2OCH3 CH2CH3 N CCl E1.325 CH CH2OCH3 CH2OCH3 CH CCl
Compounds A
2b R
1 R
4 A
6 A
2a E1.326 CH CH2OCH3 CH2OCH3 N CCl E1.327 CH CH2OCH3 CH2CH2OCH3 CH CCl E1.328 CH CH2OCH3 CH2CH2OCH3 N CCl E1.329 CH CH2OCH3 CH2CN CH CCl E1.330 CH CH2OCH3 CH2CN N CCl E1.331 CH CH3 CH CCl E1.332 CH CH3 N CCl E1.333 CH CH2CH3 CH CCl E1.334 CH CH2CH3 N CCl E1.335 CH CH2OCH3 CH CCl E1.336 CH CH2OCH3 N CCl E1.337 CH CH2CH2OCH3 CH CCl E1.338 CH CH2CH2OCH3 N CCl E1.339 CH CH2CN CH CCl E1.340 CH CH2CN N CCl E1.341 CH NHAc CH3 CH CCl E1.342 CH NHAc CH3 N CCl E1.343 CH NHAc CH2CH3 CH CCl E1.344 CH NHAc CH2CH3 N CCl E1.345 CH NHAc CH2OCH3 CH CCl E1.346 CH NHAc CH2OCH3 N CCl E1.347 CH NHAc CH2CH2OCH3 CH CCl E1.348 CH NHAc CH2CH2OCH3 N CCl E1.349 CH NHAc CH2CN CH CCl E1.350 CH NHAc CH2CN N CCl E1.351 N H CH3 CH CCl E1.352 N H CH3 N CCl E1.353 N H CH2CH3 CH CCl E1.354 N H CH2CH3 N CCl E1.355 N H CH2OCH3 CH CCl E1.356 N H CH2OCH3 N CCl E1.357 N H CH2CH2OCH3 CH CCl E1.358 N H CH2CH2OCH3 N CCl E1.359 N H CH2CN CH CCl E1.360 N H CH2CN N CCl E1.361 N CH3 CH3 CH CCl
Compounds A
2b R
1 R
4 A
6 A
2a E1.362 N CH3 CH3 N CCl E1.363 N CH3 CH2CH3 CH CCl E1.364 N CH3 CH2CH3 N CCl E1.365 N CH3 CH2OCH3 CH CCl E1.366 N CH3 CH2OCH3 N CCl E1.367 N CH3 CH2CH2OCH3 CH CCl E1.368 N CH3 CH2CH2OCH3 N CCl E1.369 N CH3 CH2CN CH CCl E1.370 N CH3 CH2CN N CCl E1.371 N CH2OCH3 CH3 CH CCl E1.372 N CH2OCH3 CH3 N CCl E1.373 N CH2OCH3 CH2CH3 CH CCl E1.374 N CH2OCH3 CH2CH3 N CCl E1.375 N CH2OCH3 CH2OCH3 CH CCl E1.376 N CH2OCH3 CH2OCH3 N CCl E1.377 N CH2OCH3 CH2CH2OCH3 CH CCl E1.378 N CH2OCH3 CH2CH2OCH3 N CCl E1.379 N CH2OCH3 CH2CN CH CCl E1.380 N CH2OCH3 CH2CN N CCl E1.381 N CH3 CH CCl E1.382 N CH3 N CCl E1.383 N CH2CH3 CH CCl E1.384 N CH2CH3 N CCl E1.385 N CH2OCH3 CH CCl E1.386 N CH2OCH3 N CCl E1.387 N CH2CH2OCH3 CH CCl E1.388 N CH2CH2OCH3 N CCl E1.389 N CH2CN CH CCl E1.390 N CH2CN N CCl E1.391 N NHAc CH3 CH CCl E1.392 N NHAc CH3 N CCl E1.393 N NHAc CH2CH3 CH CCl E1.394 N NHAc CH2CH3 N CCl E1.395 N NHAc CH2OCH3 CH CCl E1.396 N NHAc CH2OCH3 N CCl E1.397 N NHAc CH2CH2OCH3 CH CCl
Compounds A
2b R
1 R
4 A
6 A
2a E1.398 N NHAc CH2CH2OCH3 N CCl E1.399 N NHAc CH2CN CH CCl E1.400 N NHAc CH2CN N CCl E1.401 CH H CH3 CH CCH3 E1.402 CH H CH3 N CCH3 E1.403 CH H CH2CH3 CH CCH3 E1.404 CH H CH2CH3 N CCH3 E1.405 CH H CH2OCH3 CH CCH3 E1.406 CH H CH2OCH3 N CCH3 E1.407 CH H CH2CH2OCH3 CH CCH3 E1.408 CH H CH2CH2OCH3 N CCH3 E1.409 CH H CH2CN CH CCH3 E1.410 CH H CH2CN N CCH3 E1.411 CH CH3 CH3 CH CCH3 E1.412 CH CH3 CH3 N CCH3 E1.413 CH CH3 CH2CH3 CH CCH3 E1.414 CH CH3 CH2CH3 N CCH3 E1.415 CH CH3 CH2OCH3 CH CCH3 E1.416 CH CH3 CH2OCH3 N CCH3 E1.417 CH CH3 CH2CH2OCH3 CH CCH3 E1.418 CH CH3 CH2CH2OCH3 N CCH3 E1.419 CH CH3 CH2CN CH CCH3 E1.420 CH CH3 CH2CN N CCH3 E1.421 CH CH2OCH3 CH3 CH CCH3 E1.422 CH CH2OCH3 CH3 N CCH3 E1.423 CH CH2OCH3 CH2CH3 CH CCH3 E1.424 CH CH2OCH3 CH2CH3 N CCH3 E1.425 CH CH2OCH3 CH2OCH3 CH CCH3 E1.426 CH CH2OCH3 CH2OCH3 N CCH3 E1.427 CH CH2OCH3 CH2CH2OCH3 CH CCH3 E1.428 CH CH2OCH3 CH2CH2OCH3 N CCH3 E1.429 CH CH2OCH3 CH2CN CH CCH3 E1.430 CH CH2OCH3 CH2CN N CCH3 E1.431 CH CH3 CH CCH3 E1.432 CH CH3 N CCH3 E1.433 CH CH2CH3 CH CCH3 E1.434 CH CH2CH3 N CCH3 E1.435 CH CH2OCH3 CH CCH3
Compounds A
2b R
1 R
4 A
6 A
2a E1.436 CH CH2OCH3 N CCH3 E1.437 CH CH2CH2OCH3 CH CCH3 E1.438 CH CH2CH2OCH3 N CCH3 E1.439 CH CH2CN CH CCH3 E1.440 CH CH2CN N CCH3 E1.441 CH NHAc CH3 CH CCH3 E1.442 CH NHAc CH3 N CCH3 E1.443 CH NHAc CH2CH3 CH CCH3 E1.444 CH NHAc CH2CH3 N CCH3 E1.445 CH NHAc CH2OCH3 CH CCH3 E1.446 CH NHAc CH2OCH3 N CCH3 E1.447 CH NHAc CH2CH2OCH3 CH CCH3 E1.448 CH NHAc CH2CH2OCH3 N CCH3 E1.449 CH NHAc CH2CN CH CCH3 E1.450 CH NHAc CH2CN N CCH3 E1.451 N H CH3 CH CCH3 E1.452 N H CH3 N CCH3 E1.453 N H CH2CH3 CH CCH3 E1.454 N H CH2CH3 N CCH3 E1.455 N H CH2OCH3 CH CCH3 E1.456 N H CH2OCH3 N CCH3 E1.457 N H CH2CH2OCH3 CH CCH3 E1.458 N H CH2CH2OCH3 N CCH3 E1.459 N H CH2CN CH CCH3 E1.460 N H CH2CN N CCH3 E1.461 N CH3 CH3 CH CCH3 E1.462 N CH3 CH3 N CCH3 E1.463 N CH3 CH2CH3 CH CCH3 E1.464 N CH3 CH2CH3 N CCH3 E1.465 N CH3 CH2OCH3 CH CCH3 E1.466 N CH3 CH2OCH3 N CCH3 E1.467 N CH3 CH2CH2OCH3 CH CCH3 E1.468 N CH3 CH2CH2OCH3 N CCH3 E1.469 N CH3 CH2CN CH CCH3 E1.470 N CH3 CH2CN N CCH3 E1.471 N CH2OCH3 CH3 CH CCH3 E1.472 N CH2OCH3 CH3 N CCH3 E1.473 N CH2OCH3 CH2CH3 CH CCH3
Compounds A
2b R
1 R
4 A
6 A
2a E1.474 N CH2OCH3 CH2CH3 N CCH3 E1.475 N CH2OCH3 CH2OCH3 CH CCH3 E1.476 N CH2OCH3 CH2OCH3 N CCH3 E1.477 N CH2OCH3 CH2CH2OCH3 CH CCH3 E1.478 N CH2OCH3 CH2CH2OCH3 N CCH3 E1.479 N CH2OCH3 CH2CN CH CCH3 E1.480 N CH2OCH3 CH2CN N CCH3 E1.481 N CH3 CH CCH3 E1.482 N CH3 N CCH3 E1.483 N CH2CH3 CH CCH3 E1.484 N CH2CH3 N CCH3 E1.485 N CH2OCH3 CH CCH3 E1.486 N CH2OCH3 N CCH3 E1.487 N CH2CH2OCH3 CH CCH3 E1.488 N CH2CH2OCH3 N CCH3 E1.489 N CH2CN CH CCH3 E1.490 N CH2CN N CCH3 E1.491 N NHAc CH3 CH CCH3 E1.492 N NHAc CH3 N CCH3 E1.493 N NHAc CH2CH3 CH CCH3 E1.494 N NHAc CH2CH3 N CCH3 E1.495 N NHAc CH2OCH3 CH CCH3 E1.496 N NHAc CH2OCH3 N CCH3 E1.497 N NHAc CH2CH2OCH3 CH CCH3 E1.498 N NHAc CH2CH2OCH3 N CCH3 E1.499 N NHAc CH2CN CH CCH3 E1.500 N NHAc CH2CN N CCH3 E1.501 CH H CH3 CH CCH2CH3 E1.502 CH H CH3 N CCH2CH3 E1.503 CH H CH2CH3 CH CCH2CH3 E1.504 CH H CH2CH3 N CCH2CH3 E1.505 CH H CH2OCH3 CH CCH2CH3 E1.506 CH H CH2OCH3 N CCH2CH3 E1.507 CH H CH2CH2OCH3 CH CCH2CH3 E1.508 CH H CH2CH2OCH3 N CCH2CH3 E1.509 CH H CH2CN CH CCH2CH3
Compounds A
2b R
1 R
4 A
6 A
2a E1.510 CH H CH2CN N CCH2CH3 E1.511 CH CH3 CH3 CH CCH2CH3 E1.512 CH CH3 CH3 N CCH2CH3 E1.513 CH CH3 CH2CH3 CH CCH2CH3 E1.514 CH CH3 CH2CH3 N CCH2CH3 E1.515 CH CH3 CH2OCH3 CH CCH2CH3 E1.516 CH CH3 CH2OCH3 N CCH2CH3 E1.517 CH CH3 CH2CH2OCH3 CH CCH2CH3 E1.518 CH CH3 CH2CH2OCH3 N CCH2CH3 E1.519 CH CH3 CH2CN CH CCH2CH3 E1.520 CH CH3 CH2CN N CCH2CH3 E1.521 CH CH2OCH3 CH3 CH CCH2CH3 E1.522 CH CH2OCH3 CH3 N CCH2CH3 E1.523 CH CH2OCH3 CH2CH3 CH CCH2CH3 E1.524 CH CH2OCH3 CH2CH3 N CCH2CH3 E1.525 CH CH2OCH3 CH2OCH3 CH CCH2CH3 E1.526 CH CH2OCH3 CH2OCH3 N CCH2CH3 E1.527 CH CH2OCH3 CH2CH2OCH3 CH CCH2CH3 E1.528 CH CH2OCH3 CH2CH2OCH3 N CCH2CH3 E1.529 CH CH2OCH3 CH2CN CH CCH2CH3 E1.530 CH CH2OCH3 CH2CN N CCH2CH3 E1.531 CH CH3 CH CCH2CH3 E1.532 CH CH3 N CCH2CH3 E1.533 CH CH2CH3 CH CCH2CH3 E1.534 CH CH2CH3 N CCH2CH3 E1.535 CH CH2OCH3 CH CCH2CH3 E1.536 CH CH2OCH3 N CCH2CH3 E1.537 CH CH2CH2OCH3 CH CCH2CH3 E1.538 CH CH2CH2OCH3 N CCH2CH3 E1.539 CH CH2CN CH CCH2CH3 E1.540 CH CH2CN N CCH2CH3 E1.541 CH NHAc CH3 CH CCH2CH3 E1.542 CH NHAc CH3 N CCH2CH3 E1.543 CH NHAc CH2CH3 CH CCH2CH3 E1.544 CH NHAc CH2CH3 N CCH2CH3 E1.545 CH NHAc CH2OCH3 CH CCH2CH3
Compounds A
2b R
1 R
4 A
6 A
2a E1.546 CH NHAc CH2OCH3 N CCH2CH3 E1.547 CH NHAc CH2CH2OCH3 CH CCH2CH3 E1.548 CH NHAc CH2CH2OCH3 N CCH2CH3 E1.549 CH NHAc CH2CN CH CCH2CH3 E1.550 CH NHAc CH2CN N CCH2CH3 E1.551 N H CH3 CH CCH2CH3 E1.552 N H CH3 N CCH2CH3 E1.553 N H CH2CH3 CH CCH2CH3 E1.554 N H CH2CH3 N CCH2CH3 E1.555 N H CH2OCH3 CH CCH2CH3 E1.556 N H CH2OCH3 N CCH2CH3 E1.557 N H CH2CH2OCH3 CH CCH2CH3 E1.558 N H CH2CH2OCH3 N CCH2CH3 E1.559 N H CH2CN CH CCH2CH3 E1.560 N H CH2CN N CCH2CH3 E1.561 N CH3 CH3 CH CCH2CH3 E1.562 N CH3 CH3 N CCH2CH3 E1.563 N CH3 CH2CH3 CH CCH2CH3 E1.564 N CH3 CH2CH3 N CCH2CH3 E1.565 N CH3 CH2OCH3 CH CCH2CH3 E1.566 N CH3 CH2OCH3 N CCH2CH3 E1.567 N CH3 CH2CH2OCH3 CH CCH2CH3 E1.568 N CH3 CH2CH2OCH3 N CCH2CH3 E1.569 N CH3 CH2CN CH CCH2CH3 E1.570 N CH3 CH2CN N CCH2CH3 E1.571 N CH2OCH3 CH3 CH CCH2CH3 E1.572 N CH2OCH3 CH3 N CCH2CH3 E1.573 N CH2OCH3 CH2CH3 CH CCH2CH3 E1.574 N CH2OCH3 CH2CH3 N CCH2CH3 E1.575 N CH2OCH3 CH2OCH3 CH CCH2CH3 E1.576 N CH2OCH3 CH2OCH3 N CCH2CH3 E1.577 N CH2OCH3 CH2CH2OCH3 CH CCH2CH3 E1.578 N CH2OCH3 CH2CH2OCH3 N CCH2CH3 E1.579 N CH2OCH3 CH2CN CH CCH2CH3 E1.580 N CH2OCH3 CH2CN N CCH2CH3 E1.581 N CH3 CH CCH2CH3 E1.582 N CH3 N CCH2CH3 E1.583 N CH2CH3 CH CCH2CH3 E1.584 N CH2CH3 N CCH2CH3
Compounds A
2b R
1 R
4 A
6 A
2a E1.585 N CH2OCH3 CH CCH2CH3 E1.586 N CH2OCH3 N CCH2CH3 E1.587 N CH2CH2OCH3 CH CCH2CH3 E1.588 N CH2CH2OCH3 N CCH2CH3 E1.589 N CH2CN CH CCH2CH3 E1.590 N CH2CN N CCH2CH3 E1.591 N NHAc CH3 CH CCH2CH3 E1.592 N NHAc CH3 N CCH2CH3 E1.593 N NHAc CH2CH3 CH CCH2CH3 E1.594 N NHAc CH2CH3 N CCH2CH3 E1.595 N NHAc CH2OCH3 CH CCH2CH3 E1.596 N NHAc CH2OCH3 N CCH2CH3 E1.597 N NHAc CH2CH2OCH3 CH CCH2CH3 E1.598 N NHAc CH2CH2OCH3 N CCH2CH3 E1.599 N NHAc CH2CN CH CCH2CH3 E1.600 N NHAc CH2CN N CCH2CH3 E1.601 CH H CH3 CH CCN E1.602 CH H CH3 N CCN E1.603 CH H CH2CH3 CH CCN E1.604 CH H CH2CH3 N CCN E1.605 CH H CH2OCH3 CH CCN E1.606 CH H CH2OCH3 N CCN E1.607 CH H CH2CH2OCH3 CH CCN E1.608 CH H CH2CH2OCH3 N CCN E1.609 CH H CH2CN CH CCN E1.610 CH H CH2CN N CCN E1.611 CH CH3 CH3 CH CCN E1.612 CH CH3 CH3 N CCN E1.613 CH CH3 CH2CH3 CH CCN E1.614 CH CH3 CH2CH3 N CCN E1.615 CH CH3 CH2OCH3 CH CCN E1.616 CH CH3 CH2OCH3 N CCN E1.617 CH CH3 CH2CH2OCH3 CH CCN E1.618 CH CH3 CH2CH2OCH3 N CCN E1.619 CH CH3 CH2CN CH CCN E1.620 CH CH3 CH2CN N CCN E1.621 CH CH2OCH3 CH3 CH CCN E1.622 CH CH2OCH3 CH3 N CCN
Compounds A
2b R
1 R
4 A
6 A
2a E1.623 CH CH2OCH3 CH2CH3 CH CCN E1.624 CH CH2OCH3 CH2CH3 N CCN E1.625 CH CH2OCH3 CH2OCH3 CH CCN E1.626 CH CH2OCH3 CH2OCH3 N CCN E1.627 CH CH2OCH3 CH2CH2OCH3 CH CCN E1.628 CH CH2OCH3 CH2CH2OCH3 N CCN E1.629 CH CH2OCH3 CH2CN CH CCN E1.630 CH CH2OCH3 CH2CN N CCN E1.631 CH CH3 CH CCN E1.632 CH CH3 N CCN E1.633 CH CH2CH3 CH CCN E1.634 CH CH2CH3 N CCN E1.635 CH CH2OCH3 CH CCN E1.636 CH CH2OCH3 N CCN E1.637 CH CH2CH2OCH3 CH CCN E1.638 CH CH2CH2OCH3 N CCN E1.639 CH CH2CN CH CCN E1.640 CH CH2CN N CCN E1.641 CH NHAc CH3 CH CCN E1.642 CH NHAc CH3 N CCN E1.643 CH NHAc CH2CH3 CH CCN E1.644 CH NHAc CH2CH3 N CCN E1.645 CH NHAc CH2OCH3 CH CCN E1.646 CH NHAc CH2OCH3 N CCN E1.647 CH NHAc CH2CH2OCH3 CH CCN E1.648 CH NHAc CH2CH2OCH3 N CCN E1.649 CH NHAc CH2CN CH CCN E1.650 CH NHAc CH2CN N CCN E1.651 N H CH3 CH CCN E1.652 N H CH3 N CCN E1.653 N H CH2CH3 CH CCN E1.654 N H CH2CH3 N CCN E1.655 N H CH2OCH3 CH CCN E1.656 N H CH2OCH3 N CCN E1.657 N H CH2CH2OCH3 CH CCN E1.658 N H CH2CH2OCH3 N CCN
Compounds A
2b R
1 R
4 A
6 A
2a E1.659 N H CH2CN CH CCN E1.660 N H CH2CN N CCN E1.661 N CH3 CH3 CH CCN E1.662 N CH3 CH3 N CCN E1.663 N CH3 CH2CH3 CH CCN E1.664 N CH3 CH2CH3 N CCN E1.665 N CH3 CH2OCH3 CH CCN E1.666 N CH3 CH2OCH3 N CCN E1.667 N CH3 CH2CH2OCH3 CH CCN E1.668 N CH3 CH2CH2OCH3 N CCN E1.669 N CH3 CH2CN CH CCN E1.670 N CH3 CH2CN N CCN E1.671 N CH2OCH3 CH3 CH CCN E1.672 N CH2OCH3 CH3 N CCN E1.673 N CH2OCH3 CH2CH3 CH CCN E1.674 N CH2OCH3 CH2CH3 N CCN E1.675 N CH2OCH3 CH2OCH3 CH CCN E1.676 N CH2OCH3 CH2OCH3 N CCN E1.677 N CH2OCH3 CH2CH2OCH3 CH CCN E1.678 N CH2OCH3 CH2CH2OCH3 N CCN E1.679 N CH2OCH3 CH2CN CH CCN E1.680 N CH2OCH3 CH2CN N CCN E1.681 N CH3 CH CCN E1.682 N CH3 N CCN E1.683 N CH2CH3 CH CCN E1.684 N CH2CH3 N CCN E1.685 N CH2OCH3 CH CCN E1.686 N CH2OCH3 N CCN E1.687 N CH2CH2OCH3 CH CCN E1.688 N CH2CH2OCH3 N CCN E1.689 N CH2CN CH CCN E1.690 N CH2CN N CCN E1.691 N NHAc CH3 CH CCN E1.692 N NHAc CH3 N CCN E1.693 N NHAc CH2CH3 CH CCN E1.694 N NHAc CH2CH3 N CCN
Compounds A
2b R
1 R
4 A
6 A
2a E1.695 N NHAc CH2OCH3 CH CCN E1.696 N NHAc CH2OCH3 N CCN E1.697 N NHAc CH2CH2OCH3 CH CCN E1.698 N NHAc CH2CH2OCH3 N CCN E1.699 N NHAc CH2CN CH CCN E1.700 N NHAc CH2CN N CCN Table 1.2 provides 700 compounds E2.1 to E2.700 of formula (Ia) wherein A is CH, R
2 is H, R
5 is CH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.3 provides 700 compounds E3.1 to E3.700 of formula (Ia) wherein A is CH, R
2 is H, R
5 is OCH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.4 provides 700 compounds E4.1 to E4.700 of formula (Ia) wherein A is CH, R
2 is H, R
5 is OCH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.5 provides 700 compounds E5.1 to E5.700 of formula (Ia) wherein A is CH, R
2 is H, R
5 is
, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.6 provides 700 compounds E6.1 to E6.700 of formula (Ia) wherein A is CH, R
2 is H, R
5 is
, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.7 provides 700 compounds E7.1 to E7.700 of formula (Ia) wherein A is CH, R
2 is CH3, R
5 is CH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.8 provides 700 compounds E8.1 to E8.700 of formula (Ia) wherein A is CH, R
2 is CH3, R
5 is CH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.9 provides 700 compounds E9.1 to E9.700 of formula (Ia) wherein A is CH, R
2 is CH3, R
5 is OCH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.10 provides 700 compounds E10.1 to E10.700 of formula (Ia) wherein A is CH, R
2 is CH3, R
5 is OCH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.11 provides 700 compounds E11.1 to E11.700 of formula (Ia) wherein A is CH, R
2 is CH3, R
5 is , A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.12 provides 700 compounds E12.1 to E12.700 of formula (Ia) wherein A is CH, R
2 is CH3, R
5 is , A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.13 provides 700 compounds E13.1 to E13.700 of formula (Ia) wherein A is CH, R
2 is CH2CH3, R
5 is CH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.14 provides 700 compounds E14.1 to E14.700 of formula (Ia) wherein A is CH, R
2 is CH2CH3, R
5 is CH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.15 provides 700 compounds E15.1 to E15.700 of formula (Ia) wherein A is CH, R
2 is CH2CH3, R
5 is OCH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.16 provides 700 compounds E16.1 to E16.700 of formula (Ia) wherein A is CH, R
2 is CH2CH3, R
5 is OCH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z.
Table 1.17 provides 700 compounds E17.1 to E17.700 of formula (Ia) wherein A is CH, R
2 is CH2CH3,
are as defined in table Z. Table 1.18 provides 700 compounds E18.1 to E18.700 of formula (Ia) wherein A is CH, R
2 is CH2CH3,
are as defined in table Z. Table 1.19 provides 700 compounds E19.1 to E19.700 of formula (Ia) wherein A is CH, R
2 is F, R
5 is CH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.20 provides 700 compounds E20.1 to E20.700 of formula (Ia) wherein A is CH, R
2 is F, R
5 is CH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.21 provides 700 compounds E21.1 to E21.700 of formula (Ia) wherein A is CH, R
2 is F, R
5 is OCH
3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.22 provides 700 compounds E22.1 to E22.700 of formula (Ia) wherein A is CH, R
2 is F, R
5 is OCH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.23 provides 700 compounds E23.1 to E23.700 of formula (Ia) wherein A is CH, R
2 is F, R
5 is , A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.24 provides 700 compounds E24.1 to E24.700 of formula (Ia) wherein A is CH, R
2 is F, R
5R
5 is , A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.25 provides 700 compounds E25.1 to E25.700 of formula (Ia) wherein A is CH, R
2 is Cl, R
5 is CH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.26 provides 700 compounds E26.1 to E26.700 of formula (Ia) wherein A is CH, R
2R
2 is Cl, R
5 is CH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.27 provides 700 compounds E27.1 to E27.700 of formula (Ia) wherein A is CH, R
2 is Cl, R
5 is OCH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.28 provides 700 compounds E28.1 to E28.700 of formula (Ia) wherein A is CH, R
2 is Cl, R
5 is OCH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.29 provides 700 compounds E29.1 to E29.700 of formula (Ia) wherein A is CH, R
2 is Cl, R
5 is , A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.30 provides 700 compounds E30.1 to E30.700 of formula (Ia) wherein A is CH, R
2 is Cl, R
5 is , A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.31 provides 700 compounds E31.1 to E31.700 of formula (Ia) wherein A is CH, R
2 is Br, R
5 is CH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.32 provides 700 compounds E32.1 to E32.700 of formula (Ia) wherein A is CH, R
2 is Br, R
5 is CH
3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.33 provides 700 compounds E33.1 to E33.700 of formula (Ia) wherein A is CH, R
2 is Br, R
5 is OCH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.34 provides 700 compounds E34.1 to E34.700 of formula (Ia) wherein A is CH, R
2 is Br, R
5 is OCH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.35 provides 700 compounds E35.1 to E35.700 of formula (Ia) wherein A is CH, R
2 is Br, R
5 is , A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z.
Table 1.36 provides 700 compounds E36.1 to E36.700 of formula (Ia) wherein A is CH, R
2 is Br, R
5 is , A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.37 provides 700 compounds E37.1 to E37.700 of formula (Ia) wherein A is CH, R
2 is CN, R
5 is CH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.38 provides 700 compounds E38.1 to E38.700 of formula (Ia) wherein A is CH, R
2 is CN, R
5 is CH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.39 provides 700 compounds E39.1 to E39.700 of formula (Ia) wherein A is CH, R
2 is CN, R
5 is OCH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.40 provides 700 compounds E40.1 to E40.700 of formula (Ia) wherein A is CH, R
2 is CN, R
5 is OCH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.41 provides 700 compounds E41.1 to E41.700 of formula (Ia) wherein A is CH, R
2 is CN, R
5 is , A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.42 provides 700 compounds E42.1 to E42.700 of formula (Ia) wherein A is CH, R
2 is CN, R
5 is , A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.43 provides 700 compounds E43.1 to E43.700 of formula (Ia) wherein A is CH, R
2 is OCH3, R
5 is CH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.44 provides 700 compounds E44.1 to E44.700 of formula (Ia) wherein A is CH, R
2 is OCH3, R5 is CH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.45 provides 700 compounds E45.1 to E45.700 of formula (Ia) wherein A is CH, R
2 is OCH3, R5 is OCH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.46 provides 700 compounds E46.1 to E46.700 of formula (Ia) wherein A is CH, R
2 is OCH3, R5 is OCH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.47 provides 700 compounds E47.1 to E47.700 of formula (Ia) wherein A is CH, R
2 is OCH3, R5
are as defined in table Z. Table 1.48 provides 700 compounds E48.1 to E48.700 of formula (Ia) wherein A is CH, R
2 is OCH3, R
5
are as defined in table Z. Table 1.49 provides 700 compounds E49.1 to E49.700 of formula (Ia) wherein A is CH, R
2 is OCH2CH3, R
5R
5 is CH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.50 provides 700 compounds E50.1 to E50.700 of formula (Ia) wherein A is CH, R
2 is OCH2CH3, R
5 is CH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.51 provides 700 compounds E51.1 to E51.700 of formula (Ia) wherein A is CH, R
2 is OCH2CH3, R
5 is OCH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.52 provides 700 compounds E52.1 to E52.700 of formula (Ia) wherein A is CH, R
2 is OCH2CH3, R
5 is OCH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.53 provides 700 compounds E53.1 to E53.700 of formula (Ia) wherein A is CH, R
2 is OCH2CH3,
are as defined in table Z. Table 1.54 provides 700 compounds E54.1 to E54.700 of formula (Ia) wherein A is CH, R
2 is OCH2CH3, R
5 is , A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z.
Table 1.55 provides 700 compounds E55.1 to E55.700 of formula (Ia) wherein A is CH, R
2 is OCH2CH2OCH3, R
5 is CH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.56 provides 700 compounds E56.1 to E56.700 of formula (Ia) wherein A is CH, R
2 is OCH2CH2OCH3, R
5 is CH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.57 provides 700 compounds E57.1 to E57.700 of formula (Ia) wherein A is CH, R
2 is OCH2CH2OCH3, R
5 is OCH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.58 provides 700 compounds E58.1 to E58.700 of formula (Ia) wherein A is CH, R
2 is OCH2CH2OCH3, R
5 is OCH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.59 provides 700 compounds E59.1 to E59.700 of formula (Ia) wherein A is CH, R
2 is
are as defined in table Z. Table 1.60 provides 700 compounds E60.1 to E60.700 of formula (Ia) wherein A is CH, R
2 is
are as defined in table Z. Table 1.61 provides 700 compounds E61.1 to E61.700 of formula (Ia) wherein A is CH, R
2 is OH, R
5 is CH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.62 provides 700 compounds E62.1 to E62.700 of formula (Ia) wherein A is CH, R
2 is OH, R
5 is CH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.63 provides 700 compounds E63.1 to E63.700 of formula (Ia) wherein A is CH, R
2 is OH, R
5 is OCH
3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.64 provides 700 compounds E64.1 to E64.700 of formula (Ia) wherein A is CH, R
2 is OH, R
5R
5 is OCH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.65 provides 700 compounds E65.1 to E65.700 of formula (Ia) wherein A is CH, R
2 is OH, R
5 is , A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.66 provides 700 compounds E66.1 to E66.700 of formula (Ia) wherein A is CH, R
2 is OH, R
5 is , A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.67 provides 700 compounds E67.1 to E67.700 of formula (Ia) wherein A is N, R
2 is H, R
5 is CH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.68 provides 700 compounds E68.1 to E68.700 of formula (Ia) wherein A is N, R
2 is H, R
5 is CH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.69 provides 700 compounds E69.1 to E69.700 of formula (Ia) wherein A is N, R
2 is H, R
5 is OCH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.70 provides 700 compounds E70.1 to E70.700 of formula (Ia) wherein A is N, R
2 is H, R
5 is OCH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.71 provides 700 compounds E71.1 to E71.700 of formula (Ia) wherein A is N, R
2 is H, R
5 is , A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.72 provides 700 compounds E72.1 to E72.700 of formula (Ia) wherein A is N, R
2 is H, R
5 is , A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.73 provides 700 compounds E73.1 to E73.700 of formula (Ia) wherein A is N, R
2 is CH3, R
5 is CH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z.
Table 1.74 provides 700 compounds E74.1 to E74.700 of formula (Ia) wherein A is N, R
2 is CH3, R
5 is CH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.75 provides 700 compounds E75.1 to E75.700 of formula (Ia) wherein A is N, R
2 is CH3, R
5 is OCH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.76 provides 700 compounds E76.1 to E76.700 of formula (Ia) wherein A is N, R
2 is CH3, R
5 is OCH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.77 provides 700 compounds E77.1 to E77.700 of formula (Ia) wherein A is N, R
2 is CH
3, R
5 is , A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.78 provides 700 compounds E78.1 to E78.700 of formula (Ia) wherein A is N, R
2 is CH3, R
5 is , A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.79 provides 700 compounds E79.1 to E79.700 of formula (Ia) wherein A is N, R
2 is CH2CH3, R5 is CH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.80 provides 700 compounds E80.1 to E80.700 of formula (Ia) wherein A is N, R
2 is CH2CH3, R
5R
5 is CH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.81 provides 700 compounds E81.1 to E81.700 of formula (Ia) wherein A is N, R
2 is CH2CH3, R
5 is OCH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.82 provides 700 compounds E82.1 to E82.700 of formula (Ia) wherein A is N, R
2 is CH2CH3, R5 is OCH
3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.83 provides 700 compounds E83.1 to E83.700 of formula (Ia) wherein A is N, R
2 is CH2CH3, R5
are as defined in table Z. Table 1.84 provides 700 compounds E84.1 to E84.700 of formula (Ia) wherein A is N, R
2 is CH2CH3, R5
are as defined in table Z. Table 1.85 provides 700 compounds E85.1 to E85.700 of formula (Ia) wherein A is N, R
2 is F, R
5 is CH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.86 provides 700 compounds E86.1 to E86.700 of formula (Ia) wherein A is N, R
2 is F, R
5 is CH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.87 provides 700 compounds E87.1 to E87.700 of formula (Ia) wherein A is N, R
2 is F, R
5 is OCH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.88 provides 700 compounds E88.1 to E88.700 of formula (Ia) wherein A is N, R
2 is F, R
5 is OCH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.89 provides 700 compounds E89.1 to E89.700 of formula (Ia) wherein A is N, R
2 is F, R
5 is , A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.90 provides 700 compounds E90.1 to E90.700 of formula (Ia) wherein A is N, R
2 is F, R
5 is , A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.91 provides 700 compounds E91.1 to E91.700 of formula (Ia) wherein A is N, R
2 is Cl, R
5 is CH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.92 provides 700 compounds E92.1 to E92.700 of formula (Ia) wherein A is N, R
2 is Cl, R
5 is CH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z.
Table 1.93 provides 700 compounds E93.1 to E93.700 of formula (Ia) wherein A is N, R
2 is Cl, R
5 is OCH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.94 provides 700 compounds E94.1 to E94.700 of formula (Ia) wherein A is N, R
2 is Cl, R
5 is OCH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.95 provides 700 compounds E95.1 to E95.700 of formula (Ia) wherein A is N, R
2 is Cl, R
5 is , A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.96 provides 700 compounds E96.1 to E96.700 of formula (Ia) wherein A is N, R
2 is Cl, R
5 is , A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.97 provides 700 compounds E97.1 to E97.700 of formula (Ia) wherein A is N, R
2 is Br, R
5 is CH
3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.98 provides 700 compounds E98.1 to E98.700 of formula (Ia) wherein A is N, R
2 is Br, R
5 is CH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.99 provides 700 compounds E99.1 to E99.700 of formula (Ia) wherein A is N, R
2 is Br, R
5 is OCH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.100 provides 700 compounds E100.1 to E100.700 of formula (Ia) wherein A is N, R
2 is Br, R
5 is OCH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.101 provides 700 compounds E101.1 to E101.700 of formula (Ia) wherein A is N, R
2 is Br, R
5 is , A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.102 provides 700 compounds E102.1 to E102.700 of formula (Ia) wherein A is N, R
2 is Br, R
5 is , A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.103 provides 700 compounds E103.1 to E103.700 of formula (Ia) wherein A is N, R
2 is CN, R
5 is CH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.104 provides 700 compounds E104.1 to E104.700 of formula (Ia) wherein A is N, R
2 is CN, R
5 is CH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.105 provides 700 compounds E105.1 to E105.700 of formula (Ia) wherein A is N, R
2 is CN, R
5 is OCH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.106 provides 700 compounds E106.1 to E106.700 of formula (Ia) wherein A is N, R
2 is CN, R
5 is OCH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.107 provides 700 compounds E107.1 to E107.700 of formula (Ia) wherein A is N, R
2 is CN, R
5
are as defined in table Z. Table 1.108 provides 700 compounds E108.1 to E108.700 of formula (Ia) wherein A is N, R
2 is CN, R
5
are as defined in table Z. Table 1.109 provides 700 compounds E109.1 to E109.700 of formula (Ia) wherein A is N, R
2 is OCH3, R
5 is CH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.110 provides 700 compounds E110.1 to E110.700 of formula (Ia) wherein A is N, R
2 is OCH3, R
5 is CH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.111 provides 700 compounds E111.1 to E111.700 of formula (Ia) wherein A is N, R
2 is OCH3, R
5 is OCH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z.
Table 1.112 provides 700 compounds E112.1 to E112.700 of formula (Ia) wherein A is N, R
2 is OCH3, R
5 is OCH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.113 provides 700 compounds E113.1 to E113.700 of formula (Ia) wherein A is N, R
2 is OCH3,
table Z. Table 1.114 provides 700 compounds E114.1 to E114.700 of formula (Ia) wherein A is N,
are as defined in table Z. Table 1.115 provides 700 compounds E115.1 to E115.700 of formula (Ia) wherein A is N, R
2 is OCH2CH3, R
5 is CH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.116 provides 700 compounds E116.1 to E116.700 of formula (Ia) wherein A is N, R
2 is OCH
2CH
3, R
5 is CH
3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.117 provides 700 compounds E117.1 to E117.700 of formula (Ia) wherein A is N, R
2 is OCH2CH3, R
5 is OCH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.118 provides 700 compounds E118.1 to E118.700 of formula (Ia) wherein A is N, R
2 is OCH2CH3, R
5 is OCH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.119 provides 700 compounds E119.1 to E119.700 of formula (Ia) wherein A is N, R
2 is
are as defined in table Z. Table 1.120 provides 700 compounds E120.1 to E120.700 of formula (Ia) wherein A is N, R
2 is
are as defined in table Z. Table 1.121 provides 700 compounds E121.1 to E121.700 of formula (Ia) wherein A is N, R
2 is OCH2CH2OCH3, R
5 is CH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.122 provides 700 compounds E122.1 to E122.700 of formula (Ia) wherein A is N, R
2 is OCH2CH2OCH3, R
5 is CH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.123 provides 700 compounds E123.1 to E123.700 of formula (Ia) wherein A is N, R
2 is OCH2CH2OCH3, R
5 is OCH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.124 provides 700 compounds E124.1 to E124.700 of formula (Ia) wherein A is N, R
2 is OCH2CH2OCH3, R
5 is OCH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.125 provides 700 compounds E125.1 to E125.700 of formula (Ia) wherein A is N, R
2 is
are as defined in table Z. Table 1.126 provides 700 compounds E126.1 to E126.700 of formula (Ia) wherein A is N, R
2 is
are as defined in table Z. Table 1.127 provides 700 compounds E127.1 to E127.700 of formula (Ia) wherein A is N, R
2 is OH, R
5 is CH
3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.128 provides 700 compounds E128.1 to E128.700 of formula (Ia) wherein A is N, R
2 is OH, R
5 is CH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.129 provides 700 compounds E129.1 to E129.700 of formula (Ia) wherein A is N, R
2R
2 is OH, R
5 is OCH3, A
7 is CH and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z. Table 1.130 provides 700 compounds E130.1 to E130.700 of formula (Ia) wherein A is N, R
2 is OH, R
5 is OCH3, A
7 is N and A
2a, A
2b, R
1, R
4, A
6 are as defined in table Z.
Table 1.131 provides 700 compounds E131.1 to E131.700 of formula (Ia) wherein A is N, R
2 is OH, R
5
are as defined in table Z. Table 1.132 provides 700 compounds E132.1 to E132.700 of formula (Ia) wherein A is N, R
2 is OH, R
5
are as defined in table Z. Compounds according to the invention may possess any number of benefits including, inter alia, advantageous levels of biological activity for protecting plants against diseases that are caused by fungi or superior properties for use as agrochemical active ingredients (for example, greater biological activity, an advantageous spectrum of activity, an increased safety profile, improved physico-chemical properties, or increased biodegradability). Compounds according to the invention have particularly advantageous levels of biological activity for protecting plants against oomycetes such as Phytophthora, Plasmopara and Pythium. Compounds of formula (I), can be made as shown in the following schemes 1 to 22, in which, unless otherwise stated, the definition of each variable is as defined in the present invention. Compounds of formula (I) can be prepared by the reaction of a compound of formula (II) with a compound of formula (III), wherein either R
8 is independently from each other hydrogen, C
1-C
6 alkyl or wherein two R
8 together can form a C3-C8 cycloalkyl, in the presence of a base, such as triethylamine, diisopropylethylamine, pyridine, Cs2CO3, K2CO3, K2HPO4 or NaOtBu, and a suitable catalyst, such as copper(II) acetate, copper(II) carbonate, copper(II) hydroxide, copper oxide, copper(I) iodide or copper(I) bromide, in a suitable solvent, such as dimethylformamide, dimethylacetamide, dioxane, acetonitrile, tetrahydrofuran or toluene. In some instances, the outcome of the reaction can be improved by adding boric acid or molecular sieves to the reaction mixture. It is understood that these transformations are done under air as oxygen is needed as a terminal oxidant. In some cases, the copper species might be used in stochiometric amount. This transformation is depicted in Scheme 1.

Alternatively, compounds of formula (I) can be prepared by the reaction of a compound of formula (II) with a compound of formula (IV), wherein X is Cl, Br or I, in the presence of a base, such as Cs2CO3, K2CO3, K2HPO4 or NaOtBu, and a suitable palladium catalyst, such as XPhos Pd G3, t-BuXPhos Pd
G3, Me4tBuXPhos Pd G3, JohnPhos Pd G3, RuPhos Pd G3, BrettPhos Pd G3 or tBuBrettPhos Pd G3, in a suitable solvent, such as dimethylformamide, dimethylacetamide, dioxane, tetrahydrofuran or toluene. This transformation is depicted in Scheme 2.

Scheme 2 Compounds of formula (II), wherein Z is O, can be prepared by the reaction of a compound of formula (V), with a compound of formula (VI) and a coupling agent, such as N,N'-dicyclohexylcarbodiimide, bis(2- oxo-3-oxazolidinyl)phosphinic chloride, 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, propylphosphonic anhydride or cyanuric chloride, and, optionally, a base such, as triethylamine, ethyldiisopropylamine or N-methylmorpholine in a suitable solvent such ethyl acetate, dimethylformamide, tetrahydrofuran or dichloromethane. In some instance compounds of formula (V) can be used as their analogous lithium or sodium salts. This transformation is depicted in Scheme 3.

Scheme 3 Alternatively, compounds of formula (IIa), wherein Z is O, can be prepared by the reaction of a compound of formula (VII), or an hydrogen chloride salt thereof, with a compound of formula (VI) and, optionally, a base such as pyridine, 4-N,N-dimethylaminopyridine, sodium hydroxide, triethylamine, potassium carbonate, in a solvent such as tetrahydrofuran, dioxan, toluene or water. This transformation is depicted in Scheme 4.
Scheme 4 Compounds of formula (VII) can be prepared by the reaction of a compound of formula (V), with oxalyl chloride, phosgene, thionyl chloride or phosphoryl chloride in a suitable solvent such as toluene, tetrahydrofuran or dichloromethane. In some instance compounds of formula (VII) can be isolated as their hydrogen chloride salt. This transformation is depicted in Scheme 5.
(V) (VII) Scheme 5 Compounds of formula (V), are commercially available or, alternatively can be prepared by the saponification of compounds of formula (VIII), wherein R
9 is a C1-C6 alkyl, using a base, such as NaOH or LiOH, in a suitable solvent such as methanol, ethanol or water at temperature between room temperature and reflux. In some instance, compounds of formula (V) can be isolated as their sodium or lithium salt. This transformation is depicted in Scheme 6.
Scheme 6 Compounds of formula (VIII), wherein R
9 is a C1-C6 alkyl, are commercially available or, alternatively, can be prepared by the reaction of a compound of formula (IX), wherein X is Cl, Br or I, with carbon monoxide and an alcohol R
9OH, wherein R
9 is a C1-C6 alkyl, in the presence of a catalyst, such as PdCl2dppf, and, optionally, a base such as triethylamine. This transformation is depicted in scheme 7.
Compounds of formula (IX), wherein X is Cl, Br or I, are commercially available or, alternatively, compounds or formula (IXa) can be prepared by the reaction of a compound of formula (X), wherein X is Cl, Br or I with a compound of formula (XI), wherein R
9 is a C1-C6 alkyl, in the presence of an acid, such as tosylsulfonic acid, sulfuric acid or trifluoroacetic acid in a suitable solvent such as toluene, methanol, ethanol, acetonitrile or water. This transformation is depicted in scheme 8.
Scheme 8 Compounds of formula (X), wherein X is Cl, Br or I, are commercially available or, alternatively, can be prepared by the reduction of a compound of formula (XII), wherein X is Cl, Br or I or by the reduction of a compound of formula (XIII), wherein X is Cl, Br or I, with hydrogen in the presence of palladium in a suitable solvent such as tetrahydrofuran, ethanol, methanol or water, or using iron in the presence of an acid, such as hydrogen chloride, ammonium chloride or acetic acid. This transformation is depicted in scheme 9.
Scheme 9 Alternatively, compounds of formula (IIa) can be prepared by deprotection of compounds of formula (XIV), wherein R
10 is a nitrogen protecting group such as trimethylsilylethoxymethyl (SEM), tert- butoxycarbonyl (Boc) or 2-tertahydropyranyl (THP). The deprotection conditions are known to those skilled in the art. Compounds of formula (XIV) can be obtained by reaction of compounds of formula (XV), wherein X is Cl, Br or I and R
10 is a nitrogen protecting group such as trimethylsilylethoxymethyl, tert-butoxycarbonyl or 2-tertahydropyranyl, with amines of formula (VI) and carbon monoxide in the
presence of a catalyst such as [1,1-bis(diphenylphosphino)ferrocene]dichloropalladium(II) or bis(benzonitrile)palladium chloride, optionally in the presence of a ligand such as 4,5- bis(diphenylphosphino)-9,9-dimethylxanthene and, optionally, a base such as triethylamine. Compounds of formula (XV), wherein X is Cl, Br or I and R
10 is a nitrogen protecting group such as trimethylsilylethoxymethyl, tert-butoxycarbonyl or 2-tertahydropyranyl, can be prepared by protection of the nitrogen group of the imidazole ring of compounds of formula (XI), wherein X is Cl, Br or I, under conditions known to those skilled in the art. This transformation is depicted in Scheme 10.
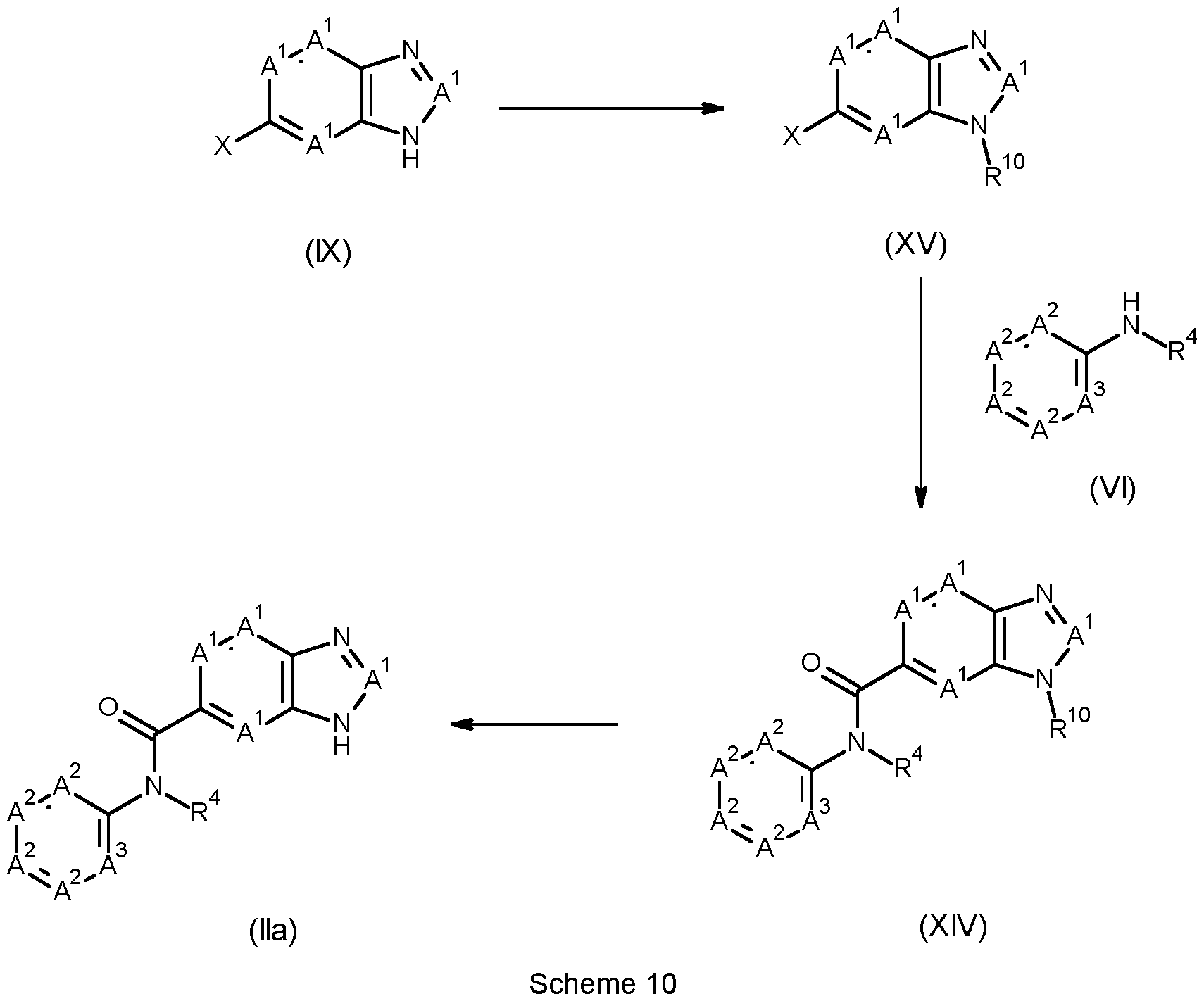
Alternatively, compounds of formula (Ia), wherein Z is O, can be prepared by the reaction of a compound of formula (XVI) with a compound of formula (VI) and a coupling agent, such as N,N'- dicyclohexylcarbodiimide, bis(2-oxo-3-oxazolidinyl)phosphinic chloride, 2-(1H-benzotriazole-1-yl)- 1,1,3,3-tetramethyluronium hexafluorophosphate, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, propylphosphonic anhydride or cyanuric chloride, and, optionally, a base such, as triethylamine, ethyldiisopropylamine or N-methylmorpholine in a suitable solvent such ethyl acetate, dimethylformamide, tetrahydrofuran or dichloromethane. In some instance compounds of formula (XVI) can be used as their analogous lithium or sodium salts. This transformation is depicted in Scheme 11.
Compounds of formula (XVI) can be prepared by the saponification of compounds of formula (XVII), wherein R
9 is a C1-C6 alkyl, using a base, such as NaOH or LiOH, in a suitable solvent such as methanol, ethanol or water at temperature between room temperature and reflux. In some instance, compounds of formula (XVII) can be isolated as their sodium or lithium salt. This transformation is depicted in Scheme 12.
Scheme 12 Compounds of formula (XVII), wherein R
9 is a C1-C6 alkyl can be prepared by the reaction of a compound of formula (XVIII), wherein X is Cl, Br or I, with carbon monoxide and an alcohol R
9OH, wherein R
9 is a C1-C6 alkyl, in the presence of a catalyst, such as PdCl2dppf, and, optionally, a base such as triethylamine. This transformation is depicted in scheme 13.
Scheme 13
Alternatively, compounds of formula (Ia), wherein Z is O, can be prepared, can be obtained from compounds of formula (Ia) can be obtained by reaction of a compound of formula (XVIII), wherein X is Cl, Br or I, with an amine of formula (VI) in the presence of carbon monoxide and in the presence of a catalyst such as [1,1-bis(diphenylphosphino)ferrocene]dichloropalladium(II) or bis(benzonitrile)palladium chloride, optionally in the presence of a ligand such as 4,5- bis(diphenylphosphino)-9,9-dimethylxanthene and, optionally, a base such as triethylamine. This transformation is depicted in Scheme 14.
Scheme 14 Compounds of formula (XVIII) can be prepared by the reaction of a compound of formula (IX) with a compound of formula (III), wherein either R
8 is independently from each other hydrogen, C1-C6 alkyl or wherein two R
8 together can form a C3-C8 cycloalkyl, in the presence of a base, such as triethylamine, diisopropylethylamine, pyridine, Cs2CO3, K2CO3, K2HPO4 or NaOtBu, and a suitable catalyst, such as copper(II) acetate, copper(II) carbonate, copper(II) hydroxide, copper oxide, copper(I) iodide or copper(I) bromide, in a suitable solvent, such as dimethylformamide, dimethylacetamide, dioxane, acetonitrile, tetrahydrofuran or toluene. In some instances, the outcome of the reaction can be improved by adding boric acid or molecular sieves to the reaction mixture. It is understood that these transformations are done under air as oxygen is needed as a terminal oxidant. In some cases, the copper species might be used in stochiometric amount. This transformation is depicted in Scheme 15.
Scheme 15
Compounds of formula (XVIIIa), wherein X is Cl, Br or I, can be prepared by the reaction of a compound of formula (XIX), wherein X is Cl, Br or I with a compound of formula (XI), wherein R
9 is a C1-C6 alkyl, in the presence of an acid, such as tosylsulfonic acid, sulfuric acid or trifluoroacetic acid in a suitable solvent such as toluene, methanol, ethanol, acetonitrile or water. This transformation is depicted in scheme 16.
Scheme 16 Compounds of formula (XIX), wherein X is Cl, Br or I, can be prepared by the reduction of a compound of formula (XX), wherein X is Cl, Br or I, with hydrogen in the presence of palladium in a suitable solvent such as tetrahydrofuran, ethanol, methanol or water, or using iron in the presence of an acid, such as hydrogen chloride, ammonium chloride or acetic acid. This transformation is depicted in scheme 17.
Scheme 17 Compounds of formula (XX), wherein X is Cl, Br or I, can be prepared by the reaction of a compound of formula (XXI), wherein X is Cl, Br or I, and wherein X
2 is F, Cl, Br or I, with a compound of formula (XXII) and a base, such as Cs2CO3, K2CO3, triethylamine, N,N-diethylethanamine or sodium tert-butoxide in a suitable solvent such as tetrahydrofuran, ethanol, methanol, acetonitrile or N,N-dimethylformamide. This transformation is depicted in scheme 18
Scheme 18 Alternatively, compounds of formula (XVII), wherein R
9 is a C1-C6 alkyl, can be prepared by reductive cyclization of a compound of formula (XXIII), wherein R
9 is a C1-C6 alkyl, with a reagent such as formic acid or methanol in the presence of a reducing agent such as Fe(0) or sodium dithionite, in a protic solvent such as methanol or propan-2-ol, eventually in the presence of an additive such as ammonium chloride. Compounds of formula (XXIII), wherein R
9 is a C1-C6 alkyl, can be obtained by reaction of a compound of formula (XXIV), wherein X
2 is F, Cl, Br or I, and R
9 is a C1-C6 alkyl, with a compound of formula (XXII) and a base, such as Cs2CO3, K2CO3, triethylamine, N,N-diethylethanamine or sodium tert-butoxide in a suitable solvent such as tetrahydrofuran, ethanol, methanol, acetonitrile or N,N- dimethylformamide. This transformation is depicted in scheme 19.
Scheme 19 Alternatively, compounds of formula (I) can be prepared by acylation of compounds of formula (XXV) with an acylation agent of formula (XXVI), wherein X is Cl or Br in the presence of a base such as
triethylamine or potassium carbonate. Compounds of formula (XXV) can be obtained by the reaction of a compound of formula (II) with a compound of formula (XXVII), wherein either R
8 is independently from each other hydrogen, C1-C6 alkyl or wherein two R
8 together can form a C3-C8 cycloalkyl, in the presence of a base, such as triethylamine, diisopropylethylamine, pyridine, Cs2CO3, K2CO3, K2HPO4 or NaOtBu, and a suitable catalyst, such as copper(II) acetate, copper(II) carbonate, copper(II) hydroxide, copper oxide, copper(I) iodide or copper(I) bromide, in a suitable solvent, such as dimethylformamide, dimethylacetamide, dioxane, acetonitrile, tetrahydrofuran or toluene. In some instances, the outcome of the reaction can be improved by adding boric acid or molecular sieves to the reaction mixture. It is understood that these transformations are done under air as oxygen is needed as a terminal oxidant. In some cases, the copper species might be used in stochiometric amount. This transformation is depicted in Scheme 20.

Scheme 20 Compounds of formula (Ib), wherein Z is S, can be prepared by the reaction of a compound of formula (Ia), wherein Z is O, with phosphorus pentasulfide or Lawesson’s reagent (CAS: 19172-47-5) in a
suitable solvent such as toluene, xylene or dichloromethane. This transformation is depicted in Scheme 21.
Scheme 21 Compounds of formula (IIb), wherein Z is S, can be prepared by the reaction of a compound of formula (IIa), wherein Z is O, with phosphorus pentasulfide or Lawesson’s reagent (CAS: 19172-47-5) in a suitable solvent such as toluene, xylene or dichloromethane. This transformation is depicted in Scheme 22.
Scheme 22 Compounds of formula (III), wherein either R
8 is independently from each other hydrogen, C1-C6 alkyl or wherein two R
8 together can form a C3-C8 cycloalkyl, and compounds of formula (XXVII), wherein either R
8 is independently from each other hydrogen, C1-C6 alkyl or wherein two R
8 together can form a C3-C8 cycloalkyl, are prepared by known methods or are commercially available. Compounds of formula (VI) are prepared by known methods or are commercially available. Compounds of formula (XI), wherein R
9 is a C1-C6 alkyl, are prepared by known methods or are commercially available. Compounds of formula (XXI), wherein X is Cl, Br or I, and wherein X
2 is F, Cl, Br or I, are prepared by known methods or are commercially available. Compounds of formula (XXII) are prepared by known methods or are commercially available.
It is understood by the person skilled in the art that the amide coupling reactions described above between an acid, an amine and a coupling agent could also be performed using the corresponding acid chloride and amine. The transformation of an acid into its corresponding acid chloride is well known by the person skilled in the art. It is also understood by the person skilled in the art that some of the reactions described above where the imidazole-ring (or triazole) nitrogen atom of the central ring is unsubstituted might work more efficiently if that imidazole (or triazole) nitrogen atom is masked with a protecting group, such as trimethylsilylethoxymethyl (SEM), tert-butoxycarbonyl (Boc) or 2-tetrahydropyranyl (THP). Protection reactions of imidazole and triazole ring with SEM, Boc or THP, along with the subsequent deprotection of those groups, are transformations well understood by the person skilled in the art. Information about those transformations can be found in literature including the Greene’s Protective Groups in Organic Synthesis, Fourth Edition, Chapter 7. When the term “compound/compounds according to the invention” is used, then this refers to compounds according to the present invention. Alternatively, the compounds according to the present invention can be obtained by using standard synthesis techniques known to the person skilled in the art. Non-exhaustive examples include oxidation reactions, reduction reactions, hydrolysis reactions, coupling reactions, aromatic nucleophilic or electrophilic substitution reactions, nucleophilic substitution reactions, nucleophilic addition reactions, olefination reactions, oxime formation, alkylation and halogenation reactions. A compound according to the present invention can be converted in a manner known per se into another compound according to the present invention by replacing one or more substituents of the starting compound according to the present invention in the customary manner by (an)other substituent(s) according to the invention. Depending on the choice of the reaction conditions and starting materials which are suitable in each case, it is possible, for example, in one reaction step only to replace one substituent by another substituent according to the invention, or a plurality of substituents can be replaced by other substituents according to the invention in the same reaction step. Salts of the compounds according to the present invention can be prepared in a manner known per se. Thus, for example, acid addition salts of the compounds according to the present invention are obtained by treatment with a suitable acid or a suitable ion exchanger reagent and salts with bases are obtained by treatment with a suitable base or with a suitable ion exchanger reagent. Salts of compounds the compounds according to the present invention can be converted in the customary manner into the free compounds, acid addition salts, for example, by treatment with a suitable basic compound or with a suitable ion exchanger reagent and salts with bases, for example, by treatment with a suitable acid or with a suitable ion exchanger reagent.
Salts of the compounds according to the present invention can be converted in a manner known per se into other salts of the compounds according to the present invention, acid addition salts, for example, into other acid addition salts, for example by treatment of a salt of inorganic acid such as hydrochloride with a suitable metal salt such as a sodium, barium or silver salt, of an acid, for example with silver acetate, in a suitable solvent in which an inorganic salt which forms, for example silver chloride, is insoluble and thus precipitates from the reaction mixture. Depending on the procedure or the reaction conditions, the compounds according to the present invention, which have salt-forming properties can be obtained in free form or in the form of salts. The compounds according to the present invention and, where appropriate, the tautomers thereof, in each case in free form or in salt form, can be present in the form of one of the stereoisomers which are possible or as a mixture of these, for example in the form of pure stereoisomers, such as antipodes and/or diastereomers, or as stereoisomer mixtures, such as enantiomer mixtures, for example racemates, diastereomer mixtures or racemate mixtures, depending on the number, absolute and relative configuration of asymmetric carbon atoms which occur in the molecule and/or depending on the configuration of non-aromatic double bonds which occur in the molecule; the invention relates to the pure stereoisomers and also to all stereoisomer mixtures which are possible and is to be understood in each case in this sense hereinabove and hereinbelow, even when stereochemical details are not mentioned specifically in each case. Diastereomer mixtures or racemate mixtures of the compounds according to the present invention, in free form or in salt form, which can be obtained depending on which starting materials and procedures have been chosen can be separated in a known manner into the pure diasteromers or racemates on the basis of the physicochemical differences of the components, for example by fractional crystallization, distillation and/or chromatography. Enantiomer mixtures, such as racemates, which can be obtained in a similar manner can be resolved into the optical antipodes by known methods, for example by recrystallization from an optically active solvent, by chromatography on chiral adsorbents, for example high-performance liquid chromatography (HPLC) on acetyl celulose, with the aid of suitable microorganisms, by cleavage with specific, immobilized enzymes, via the formation of inclusion compounds, for example using chiral crown ethers, where only one enantiomer is complexed, or by conversion into diastereomeric salts, for example by reacting a basic end-product racemate with an optically active acid, such as a carboxylic acid, for example camphor, tartaric or malic acid, or sulfonic acid, for example camphorsulfonic acid, and separating the diastereomer mixture which can be obtained in this manner, for example by fractional crystallization based on their differing solubilities, to give the diastereomers, from which the desired enantiomer can be set free by the action of suitable agents, for example basic agents. Pure diastereomers or enantiomers can be obtained according to the invention not only by separating suitable stereoisomer mixtures, but also by generally known methods of diastereoselective or
enantioselective synthesis, for example by carrying out the process according to the invention with starting materials of a suitable stereochemistry. N-oxides can be prepared by reacting a compound according to the present invention with a suitable oxidizing agent, for example the H2O2/urea adduct in the presence of an acid anhydride, e.g. trifluoroacetic anhydride. Such oxidations are known from the literature, for example from J. Med. Chem., 32 (12), 2561-73, 1989 or WO 00/15615. It is advantageous to isolate or synthesize in each case the biologically more effective stereoisomer, for example enantiomer or diastereomer, or stereoisomer mixture, for example enantiomer mixture or diastereomer mixture, if the individual components have a different biological activity. The compounds according to the present invention and, where appropriate, the tautomers thereof, in each case in free form or in salt form, can, if appropriate, also be obtained in the form of hydrates and/or include other solvents, for example those which may have been used for the crystallization of compounds which are present in solid form. The following Examples illustrate, but do not limit, the invention. The present invention also provides intermediates useful for the preparation of compounds according to the present invention. The below intermediates form a further aspect of the invention. A compound of formula (XVI)

wherein A is CH or N; A
1 are independently N or CR
1; with the proviso that no more than three A
1 are N, preferably no more than two A
1 are N, preferably no more than one A
1 is N, and more preferably the four A
1 are CR
1; R
1 are independently selected from hydrogen, hydroxy, halogen, CN, C1-6alkyl, C3-6cycloalkyl, C1- 6alkoxy-C1-6alkyl, C3-6cycloalkyl-C1-4alkyl, C1-6alkylsulfanyl, C1-6alkylsulfinyl, C1-6alkylsulfonyl, C1-6alkoxy, amino, and NHC(O)C1-6alkyl; and R
5 is selected from C1-6alkyl, C1-6alkoxy, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, C1-6alkoxyC1-6alkyl, C1- 6alkylamino, diC1-6alkylamino, and C1-6alkylC1-6alkoxyamino, wherein each of said groups is optionally substituted with one to three substituents independently selected from halogen and CN; or a salt or N-oxide thereof.
A compound of formula (XVII)
wherein A is CH or N; A
1 are independently N or CR
1; with the proviso that no more than three A
1 are N, preferably no more than two A
1 are N, preferably no more than one A
1 is N, and more preferably the four A
1 are CR
1; R
1 are independently selected from hydrogen, hydroxy, halogen, CN, C1-6alkyl, C3-6cycloalkyl, C1- 6alkoxy-C1-6alkyl, C3-6cycloalkyl-C1-4alkyl, C1-6alkylsulfanyl, C1-6alkylsulfinyl, C1-6alkylsulfonyl, C1-6alkoxy, amino, and NHC(O)C1-6alkyl; R
5 is selected from C1-6alkyl, C1-6alkoxy, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, C1-6alkoxyC1-6alkyl, C1- 6alkylamino, diC1-6alkylamino, and C1-6alkylC1-6alkoxyamino, wherein each of said groups is optionally substituted with one to three substituents independently selected from halogen and CN; and R
9 is a C1-C6 alkyl. A compound of formula (XVIII)
wherein A is CH or N; A
1 are independently N or CR
1; with the proviso that no more than three A
1 are N, preferably no more than two A
1 are N, preferably no more than one A
1 is N, and more preferably the four A
1 are CR
1; R
1 are independently selected from hydrogen, hydroxy, halogen, CN, C1-6alkyl, C3-6cycloalkyl, C1- 6alkoxy-C1-6alkyl, C3-6cycloalkyl-C1-4alkyl, C1-6alkylsulfanyl, C1-6alkylsulfinyl, C1-6alkylsulfonyl, C1-6alkoxy, amino, and NHC(O)C1-6alkyl; R
5 is selected from C1-6alkyl, C1-6alkoxy, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, C1-6alkoxyC1-6alkyl, C1- 6alkylamino, diC1-6alkylamino, and C1-6alkylC1-6alkoxyamino, wherein each of said groups is optionally substituted with one to three substituents independently selected from halogen and CN; and X is Cl, Br or I.
A compound of formula (XXIII)
wherein A is CH or N; A
1 are independently N or CR
1; with the proviso that no more than three A
1 are N, preferably no more than two A
1 are N, preferably no more than one A
1 is N, and more preferably the four A
1 are CR
1; R
1 are independently selected from hydrogen, hydroxy, halogen, CN, C1-6alkyl, C3-6cycloalkyl, C1- 6alkoxy-C1-6alkyl, C3-6cycloalkyl-C1-4alkyl, C1-6alkylsulfanyl, C1-6alkylsulfinyl, C1-6alkylsulfonyl, C1-6alkoxy, amino, and NHC(O)C1-6alkyl; R
5 is selected from C1-6alkyl, C1-6alkoxy, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, C1-6alkoxyC1-6alkyl, C1- 6alkylamino, diC1-6alkylamino, and C1-6alkylC1-6alkoxyamino, wherein each of said groups is optionally substituted with one to three substituents independently selected from halogen and CN; and R
9 is a C1-C6 alkyl. A compound of formula (XXV)
wherein A is CH or N; A
1 are independently N or CR
1; with the proviso that no more than three A
1 are N, preferably no more than two A
1 are N, preferably no more than one A
1 is N, and more preferably the four A
1 are CR
1; R
1 are independently selected from hydrogen, hydroxy, halogen, CN, C1-6alkyl, C3-6cycloalkyl, C1- 6alkoxy-C1-6alkyl, C3-6cycloalkyl-C1-4alkyl, C1-6alkylsulfanyl, C1-6alkylsulfinyl, C1-6alkylsulfonyl, C1-6alkoxy, amino, and NHC(O)C1-6alkyl; A
2 are independently CR
2 or N, with the proviso that no more than three A
2 are N, preferably no more than two A
2 are N, preferably no more than one A
2 is N, and more preferably the four A
2 are CR
2; R
2 are independently selected from hydrogen, hydroxy, halogen, CN, C1-6alkyl, C1-6alkoxy, C1-6alkoxy- C1-6alkyl, C1-6alkoxy-C1-6alkoxy, C2-6alkenyl, C2-6alkynyl, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, C1- 6alkylsulfanyl, C1-6alkylsulfinyl, C1-6alkylsulfonyl, C1-6alkoxycarbonyl, C1-6alkylaminocarbonyl, diC1- 6alkylaminocarbonyl, and C1-6alkylcarbonyl, wherein each of the C1-6alkyl, C1-6alkoxy, C1-6alkoxy-C1- 6alkyl, C1-6alkoxy-C1-6alkoxy, C2-6alkenyl, C2-6alkynyl, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, C1- 6alkylsulfanyl, C1-6alkylsulfinyl, C1-6alkylsulfonyl, C1-6alkoxycarbonyl, C1-6alkylaminocarbonyl, diC1-
6alkylaminocarbonyl, and C1-6alkylcarbonyl groups is optionally substituted with one to three substituents independently selected from halogen, hydroxy, and CN; A
3 is independently CR
3 or N; R
3 are independently selected from hydrogen, hydroxy, halogen, CN, C1-6alkyl, C1-6alkoxy, C1-6alkoxy- C1-6alkyl, C1-6alkoxy-C1-6alkoxy, C2-6alkenyl, C2-6alkynyl, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, C1- 6alkylsulfanyl, C1-6alkylsulfinyl, C1-6alkylsulfonyl, amino, C1-6alkylamino, diC1-6-alkylamino, and C3-
6cycloalkylamino, wherein each of the C
1-6alkyl, C
1-6alkoxy, C
1-6alkoxy-C
1-6alkyl, C
1-6alkoxy-C
1-6alkoxy, C2-6alkenyl, C2-6alkynyl, C3-6cycloalkyl, C3-6cycloalkyl-C1-6alkyl, C1-6alkylsulfanyl, C1-6alkylsulfinyl, C1- 6alkylsulfonyl, amino, C1-6alkylamino, diC1-6-alkylamino, and C3-6cycloalkylamino groups is optionally substituted with one to three substituents independently selected from halogen, hydroxy, and CN; R
4 is selected from C1-6alkyl, C1-6alkoxy-C1-6alkyl, C3-6cycloalkyl, C3-6cycloalkyl-C1-4alkyl, C2-6alkenyl, C2- 6alkynyl, C1-6alkoxy, C1-6alkylsulfanyl-C1-6alkyl, C1-6alkylsulfinyl-C1-6alkyl, C1-6alkylsulfonyl-C1-6alkyl, C1- 6alkoxycarbonyl-C1-6alkyl, C1-6alkylaminocarbonyl-C1-6alkyl, diC1-6alkylaminocarbonyl-C1-6alkyl, and CN, wherein each of the C1-6alkyl, C1-6alkoxy-C1-6alkyl, C3-6cycloalkyl, C3-6cycloalkyl-C1-4alkyl, C2-6alkenyl, C2-
6alkynyl, C
1-6alkoxy, C
1-6alkylsulfanyl-C
1-6alkyl, C
1-6alkylsulfinyl-C
1-6alkyl, C
1-6alkylsulfonyl-C
1-6alkyl, C
1- 6alkoxycarbonyl-C1-6alkyl, C1-6alkylaminocarbonyl-C1-6alkyl and diC1-6alkylaminocarbonyl-C1-6alkyl groups is optionally substituted with one to three substituents independently selected from halogen and CN; wherein A
3 and R
4 taken together optionally form a ring, more preferably a 5-8-membered heterocycle, more preferably a 6-membered heterocycle. The compounds of formula (I) as defined in the present invention can be used in the agricultural sector and related fields of use e.g. as active ingredients for controlling plant pathogens or on non-living materials for control of spoilage microorganisms or organisms potentially harmful to man. The novel compounds are distinguished by excellent activity at low rates of application, by being well tolerated by plants and by being environmentally safe. They have very useful curative, preventive and systemic properties and may be used for protecting numerous cultivated plants. The compounds of formula (I) as defined in the present invention can be used to inhibit or destroy the pathogens that occur on plants or parts of plants (fruit, blossoms, leaves, stems, tubers, roots) of different crops of useful plants, while at the same time protecting also those parts of the plants that grow later e.g. from phytopathogenic microorganisms. It is also possible to use compounds of formula (I) as defined in the present invention as fungicide. The term “fungicide” as used herein means a compound that controls, modifies, or prevents the growth of fungi. The term “fungicidally effective amount” means the quantity of such a compound or combination of such compounds that is capable of producing an effect on the growth of fungi. Controlling or modifying effects include all deviation from natural development, such as killing, retardation and the like, and prevention includes barrier or other defensive formation in or on a plant to prevent fungal infection. It is also possible to use compounds of formula (I) as defined in the present invention as dressing agents for the treatment of plant propagation material, e.g., seed, such as fruits, tubers or grains, or plant cuttings (for example rice), for the protection against fungal infections as well as against
phytopathogenic fungi occurring in the soil. The propagation material can be treated with a composition comprising a compound of formula (I) as defined in the present invention before planting: seed, for example, can be dressed before being sown. The compounds of formula (I) as defined in the present invention can also be applied to grains (coating), either by impregnating the seeds in a liquid formulation or by coating them with a solid formulation. The composition can also be applied to the planting site when the propagation material is being planted, for example, to the seed furrow during sowing. The invention relates also to such methods of treating plant propagation material and to the plant propagation material so treated. Furthermore the compounds of formula (I) as defined in the present invention can be used for controlling fungi in related areas, for example in the protection of technical materials, including wood and wood related technical products, in food storage, in hygiene management. In addition, the invention could be used to protect non-living materials from fungal attack, e.g. lumber, wall boards and paint. Compounds of formula (I) as defined in the present invention and fungicidal compositions containing them may be used to control plant diseases caused by a broad spectrum of fungal plant pathogens. They are effective in controlling a broad spectrum of plant diseases, such as foliar pathogens of ornamental, turf, vegetable, field, cereal, and fruit crops. These fungi and fungal vectors of disease, as well as phytopathogenic bacteria and viruses, which may be controlled are for example: Absidia corymbifera, Alternaria spp, Aphanomyces spp, Ascochyta spp, Aspergillus spp. including A. flavus, A. fumigatus, A. nidulans, A. niger, A. terrus, Aureobasidium spp. including A. pullulans, Blastomyces dermatitidis, Blumeria graminis, Bremia lactucae, Botryosphaeria spp. including B. dothidea, B. obtusa, Botrytis spp. inclusing B. cinerea, Candida spp. including C. albicans, C. glabrata, C. krusei, C. lusitaniae, C. parapsilosis, C. tropicalis, Cephaloascus fragrans, Ceratocystis spp, Cercospora spp. including C. arachidicola, Cercosporidium personatum, Cladosporium spp, Claviceps purpurea, Coccidioides immitis, Cochliobolus spp, Colletotrichum spp. including C. musae, Cryptococcus neoformans, Diaporthe spp, Didymella spp, Drechslera spp, Elsinoe spp, Epidermophyton spp, Erwinia amylovora, Erysiphe spp. including E. cichoracearum, Eutypa lata, Fusarium spp. including F. culmorum, F. graminearum, F. langsethiae, F. moniliforme, F. oxysporum, F. proliferatum, F. subglutinans, F. solani, Gaeumannomyces graminis, Gibberella fujikuroi, Gloeodes pomigena, Gloeosporium musarum, Glomerella cingulate, Guignardia bidwellii, Gymnosporangium juniperi-virginianae, Helminthosporium spp, Hemileia spp, Histoplasma spp. including H. capsulatum, Laetisaria fuciformis, Leptographium lindbergi, Leveillula taurica, Lophodermium seditiosum, Microdochium nivale, Microsporum spp, Monilinia spp, Mucor spp, Mycosphaerella spp. including M. graminicola, M. pomi, Oncobasidium theobromaeon, Ophiostoma piceae, Paracoccidioides spp, Penicillium spp. including P. digitatum, P. italicum, Petriellidium spp, Peronosclerospora spp. Including P. maydis, P. philippinensis and P. sorghi, Peronospora spp,
Phaeosphaeria nodorum, Phakopsora pachyrhizi, Phellinus igniarus, Phialophora spp, Phoma spp, Phomopsis viticola, Phytophthora spp. including P. infestans, Plasmopara spp. including P. halstedii, P. viticola, Pleospora spp., Podosphaera spp. including P. leucotricha, Polymyxa graminis, Polymyxa betae, Pseudocercosporella herpotrichoides, Pseudomonas spp, Pseudoperonospora spp. including P. cubensis, P. humuli, Pseudopeziza tracheiphila, Puccinia Spp. including P. hordei, P. recondita, P. striiformis, P. triticina, Pyrenopeziza spp, Pyrenophora spp, Pyricularia spp. including P. oryzae, Pythium spp. including P. ultimum, Ramularia spp, Rhizoctonia spp, Rhizomucor pusillus, Rhizopus arrhizus, Rhynchosporium spp, Scedosporium spp. including S. apiospermum and S. prolificans, Schizothyrium pomi, Sclerotinia spp, Sclerotium spp, Septoria spp, including S. nodorum, S. tritici, Sphaerotheca macularis, Sphaerotheca fusca (Sphaerotheca fuliginea), Sporothorix spp, Stagonospora nodorum, Stemphylium spp,. Stereum hirsutum, Thanatephorus cucumeris, Thielaviopsis basicola, Tilletia spp, Trichoderma spp. including T. harzianum, T. pseudokoningii, T. viride, Trichophyton spp, Typhula spp, Uncinula necator, Urocystis spp, Ustilago spp, Venturia spp. including V. inaequalis, Verticillium spp, and Xanthomonas spp. In particular, compounds of formula (I) as defined in the present invention and fungicidal compositions containing them may be used to control plant diseases caused by a broad spectrum of fungal plant pathogens in the Basidiomycete, Ascomycete, Oomycete and/or Deuteromycete, Blasocladiomycete, Chrytidiomycete, Glomeromycete and/or Mucoromycete classes. More particularly, the compounds of formula (I) as defined in the present invention may be used to conrol oomycetes. These pathogens may include: Oomycetes, including Phytophthora diseases such as those caused by Phytophthora capsici, Phytophthora infestans, Phytophthora sojae, Phytophthora fragariae, Phytophthora nicotianae, Phytophthora cinnamomi, Phytophthora citricola, Phytophthora citrophthora and Phytophthora erythroseptica; Pythium diseases such as those caused by Pythium aphanidermatum, Pythium arrhenomanes, Pythium graminicola, Pythium irregulare, Pythium sylvaticum and Pythium ultimum; diseases caused by Peronosporales such as Peronospora destructor, Peronospora parasitica, Plasmopara viticola, Plasmopara halstedii, Pseudoperonospora cubensis, Albugo candida, Sclerophthora macrospora and Bremia lactucae; and others such as Aphanomyces cochlioides, Labyrinthula zosterae, Peronosclerospora sorghi and Sclerospora graminicola. Ascomycetes, including blotch, spot, blast or blight diseases and/or rots for example those caused by Pleosporales such as Stemphylium solani, Stagonospora tainanensis, Spilocaea oleaginea, Setosphaeria turcica, Pyrenochaeta lycoperisici, Pleospora herbarum, Phoma destructiva, Phaeosphaeria herpotrichoides, Phaeocryptocus gaeumannii, Ophiosphaerella graminicola, Ophiobolus graminis, Leptosphaeria maculans, Hendersonia creberrima, Helminthosporium triticirepentis, Setosphaeria turcica, Drechslera glycines, Didymella bryoniae, Cycloconium oleagineum, Corynespora cassiicola, Cochliobolus sativus, Bipolaris cactivora, Venturia inaequalis, Pyrenophora teres, Pyrenophora tritici-repentis, Alternaria alternata, Alternaria brassicicola, Alternaria solani and Alternaria tomatophila, Capnodiales such as Septoria tritici, Septoria nodorum, Septoria glycines, Cercospora arachidicola, Cercospora sojina, Cercospora zeae-maydis, Cercosporella capsellae and
Cercosporella herpotrichoides, Cladosporium carpophilum, Cladosporium effusum, Passalora fulva, Cladosporium oxysporum, Dothistroma septosporum, Isariopsis clavispora, Mycosphaerella fijiensis, Mycosphaerella graminicola, Mycovellosiella koepkeii, Phaeoisariopsis bataticola, Pseudocercospora vitis, Pseudocercosporella herpotrichoides, Ramularia beticola, Ramularia collo-cygni, Magnaporthales such as Gaeumannomyces graminis, Magnaporthe grisea, Pyricularia oryzae, Diaporthales such as Anisogramma anomala, Apiognomonia errabunda, Cytospora platani, Diaporthe phaseolorum, Discula destructiva, Gnomonia fructicola, Greeneria uvicola, Melanconium juglandinum, Phomopsis viticola, Sirococcus clavigignenti-juglandacearum, Tubakia dryina, Dicarpella spp., Valsa ceratosperma, and others such as Actinothyrium graminis, Ascochyta pisi, Aspergillus flavus, Aspergillus fumigatus, Aspergillus nidulans, Asperisporium caricae, Blumeriella jaapii, Candida spp., Capnodium ramosum, Cephaloascus spp., Cephalosporium gramineum, Ceratocystis paradoxa, Chaetomium spp., Hymenoscyphus pseudoalbidus, Coccidioides spp., Cylindrosporium padi, Diplocarpon malae, Drepanopeziza campestris, Elsinoe ampelina, Epicoccum nigrum, Epidermophyton spp., Eutypa lata, Geotrichum candidum, Gibellina cerealis, Gloeocercospora sorghi, Gloeodes pomigena, Gloeosporium perennans; Gloeotinia temulenta, Griphospaeria corticola, Kabatiella lini, Leptographium microsporum, Leptosphaerulinia crassiasca, Lophodermium seditiosum, Marssonina graminicola, Microdochium nivale, Monilinia fructicola, Monographella albescens, Monosporascus cannonballus, Naemacyclus spp., Ophiostoma novo-ulmi, Paracoccidioides brasiliensis, Penicillium expansum, Pestalotia rhododendri, Petriellidium spp., Pezicula spp., Phialophora gregata, Phyllachora pomigena, Phymatotrichum omnivora, Physalospora abdita, Plectosporium tabacinum, Polyscytalum pustulans, Pseudopeziza medicaginis, Pyrenopeziza brassicae, Ramulispora sorghi, Rhabdocline pseudotsugae, Rhynchosporium secalis, Sacrocladium oryzae, Scedosporium spp., Schizothyrium pomi, Sclerotinia sclerotiorum, Sclerotinia minor; Sclerotium spp., Typhula ishikariensis, Seimatosporium mariae, Lepteutypa cupressi, Septocyta ruborum, Sphaceloma perseae, Sporonema phacidioides, Stigmina palmivora, Tapesia yallundae, Taphrina bullata, Thielviopsis basicola, Trichoseptoria fructigena, Zygophiala jamaicensis; powdery mildew diseases for example those caused by Erysiphales such as Blumeria graminis, Erysiphe polygoni, Uncinula necator, Sphaerotheca fuligena, Podosphaera leucotricha, Podospaera macularis Golovinomyces cichoracearum, Leveillula taurica, Microsphaera diffusa, Oidiopsis gossypii, Phyllactinia guttata and Oidium arachidis; molds for example those caused by Botryosphaeriales such as Dothiorella aromatica, Diplodia seriata, Guignardia bidwellii, Botrytis cinerea, Botryotinia allii, Botryotinia fabae, Fusicoccum amygdali, Lasiodiplodia theobromae, Macrophoma theicola, Macrophomina phaseolina, Phyllosticta cucurbitacearum; anthracnoses for example those caused by Glommerelales such as Colletotrichum gloeosporioides, Colletotrichum lagenarium, Colletotrichum gossypii, Glomerella cingulata, and Colletotrichum graminicola; and wilts or blights for example those caused by Hypocreales such as Acremonium strictum, Claviceps purpurea, Fusarium culmorum, Fusarium graminearum, Fusarium virguliforme, Fusarium oxysporum, Fusarium subglutinans, Fusarium oxysporum f.sp. cubense, Gerlachia nivale, Gibberella fujikuroi, Gibberella zeae, Gliocladium spp., Myrothecium verrucaria, Nectria ramulariae, Trichoderma viride, Trichothecium roseum, and Verticillium theobromae. Basidiomycetes, including smuts for example those caused by Ustilaginales such as Ustilaginoidea virens, Ustilago nuda, Ustilago tritici, Ustilago zeae, rusts for example those caused by Pucciniales such as Cerotelium fici, Chrysomyxa arctostaphyli, Coleosporium ipomoeae, Hemileia vastatrix, Puccinia
arachidis, Puccinia cacabata, Puccinia graminis, Puccinia recondita, Puccinia sorghi, Puccinia hordei, Puccinia striiformis f.sp. Hordei, Puccinia striiformis f.sp. Secalis, Pucciniastrum coryli, or Uredinales such as Cronartium ribicola, Gymnosporangium juniperi-viginianae, Melampsora medusae, Phakopsora pachyrhizi, Phragmidium mucronatum, Physopella ampelosidis, Tranzschelia discolor and Uromyces viciae-fabae; and other rots and diseases such as those caused by Cryptococcus spp., Exobasidium vexans, Marasmiellus inoderma, Mycena spp., Sphacelotheca reiliana, Typhula ishikariensis, Urocystis agropyri, Itersonilia perplexans, Corticium invisum, Laetisaria fuciformis, Waitea circinata, Rhizoctonia solani, Thanetephorus cucurmeris, Entyloma dahliae, Entylomella microspora, Neovossia moliniae and Tilletia caries. Blastocladiomycetes, such as Physoderma maydis. Mucoromycetes, such as Choanephora cucurbitarum.; Mucor spp.; Rhizopus arrhizus. As well as diseases caused by other species and genera closely related to those listed above. In addition to their fungicidal activity, the compounds and compositions comprising compounds of formula (I) as defined in the present invention may also have activity against bacteria such as Erwinia amylovora, Erwinia caratovora, Xanthomonas campestris, Pseudomonas syringae, Strptomyces scabies and other related species as well as certain protozoa. Within the scope of the present invention, target crops and/or useful plants to be protected typically comprise perennial and annual crops, such as berry plants for example blackberries, blueberries, cranberries, raspberries and strawberries; cereals for example barley, maize (corn), millet, oats, rice, rye, sorghum triticale and wheat; fibre plants for example cotton, flax, hemp, jute and sisal; field crops for example sugar and fodder beet, coffee, hops, mustard, oilseed rape (canola), poppy, sugar cane, sunflower, tea and tobacco; fruit trees for example apple, apricot, avocado, banana, cherry, citrus, nectarine, peach, pear and plum; grasses for example Bermuda grass, bluegrass, bentgrass, centipede grass, fescue, ryegrass, St. Augustine grass and Zoysia grass; herbs such as basil, borage, chives, coriander, lavender, lovage, mint, oregano, parsley, rosemary, sage and thyme; legumes for example beans, lentils, peas and soya beans; nuts for example almond, cashew, ground nut, hazelnut, peanut, pecan, pistachio and walnut; palms for example oil palm; ornamentals for example flowers, shrubs and trees; other trees, for example cacao, coconut, olive and rubber; vegetables for example asparagus, aubergine, broccoli, cabbage, carrot, cucumber, garlic, lettuce, marrow, melon, okra, onion, pepper, potato, pumpkin, rhubarb, spinach and tomato; and vines for example grapes. The useful plants and / or target crops in accordance with the invention include conventional as well as genetically enhanced or engineered varieties such as, for example, insect resistant (e.g. Bt. and VIP varieties) as well as disease resistant, herbicide tolerant (e.g. glyphosate- and glufosinate-resistant maize varieties commercially available under the trade names RoundupReady® and LibertyLink®) and nematode tolerant varieties. By way of example, suitable genetically enhanced or engineered crop varieties include the Stoneville 5599BR cotton and Stoneville 4892BR cotton varieties. The term "useful plants" and/or “target crops” is to be understood as including also useful plants that have been rendered tolerant to herbicides like bromoxynil or classes of herbicides (such as, for example,
HPPD inhibitors, ALS inhibitors, for example primisulfuron, prosulfuron and trifloxysulfuron, EPSPS (5- enol-pyrovyl-shikimate-3-phosphate-synthase) inhibitors, GS (glutamine synthetase) inhibitors or PPO (protoporphyrinogen-oxidase) inhibitors) as a result of conventional methods of breeding or genetic engineering. An example of a crop that has been rendered tolerant to imidazolinones, e.g. imazamox, by conventional methods of breeding (mutagenesis) is Clearfield® summer rape (Canola). Examples of crops that have been rendered tolerant to herbicides or classes of herbicides by genetic engineering methods include glyphosate- and glufosinate-resistant maize varieties commercially available under the trade names RoundupReady® , Herculex I® and LibertyLink®. The term "useful plants" and/or “target crops” is to be understood as including those which naturally are or have been rendered resistant to harmful insects. This includes plants transformed by the use of recombinant DNA techniques, for example, to be capable of synthesising one or more selectively acting toxins, such as are known, for example, from toxin-producing bacteria. Examples of toxins which can be expressed include ^-endotoxins, vegetative insecticidal proteins (Vip), insecticidal proteins of bacteria colonising nematodes, and toxins produced by scorpions, arachnids, wasps and fungi. An example of a crop that has been modified to express the Bacillus thuringiensis toxin is the Bt maize KnockOut ^ (Syngenta Seeds). An example of a crop comprising more than one gene that codes for insecticidal resistance and thus expresses more than one toxin is VipCot ^ (Syngenta Seeds). Crops or seed material thereof can also be resistant to multiple types of pests (so-called stacked transgenic events when created by genetic modification). For example, a plant can have the ability to express an insecticidal protein while at the same time being herbicide tolerant, for example Herculex I ^ (Dow AgroSciences, Pioneer Hi-Bred International). The term "useful plants" and/or “target crops” is to be understood as including also useful plants which have been so transformed by the use of recombinant DNA techniques that they are capable of synthesising antipathogenic substances having a selective action, such as, for example, the so-called "pathogenesis-related proteins" (PRPs, see e.g. EP-A-0392225). Examples of such antipathogenic substances and transgenic plants capable of synthesising such antipathogenic substances are known, for example, from EP-A-0392225, WO 95/33818, and EP-A-0353191. The methods of producing such transgenic plants are generally known to the person skilled in the art and are described, for example, in the publications mentioned above. Toxins that can be expressed by transgenic plants include, for example, insecticidal proteins from Bacillus cereus or Bacillus popilliae; or insecticidal proteins from Bacillus thuringiensis, such as ^- endotoxins, e.g. Cry1Ab, Cry1Ac, Cry1F, Cry1Fa2, Cry2Ab, Cry3A, Cry3Bb1 or Cry9C, or vegetative insecticidal proteins (Vip), e.g. Vip1, Vip2, Vip3 or Vip3A; or insecticidal proteins of bacteria colonising nematodes, for example Photorhabdus spp. or Xenorhabdus spp., such as Photorhabdus luminescens, Xenorhabdus nematophilus; toxins produced by animals, such as scorpion toxins, arachnid toxins, wasp toxins and other insect-specific neurotoxins; toxins produced by fungi, such as Streptomycetes toxins, plant lectins, such as pea lectins, barley lectins or snowdrop lectins; agglutinins; proteinase inhibitors, such as trypsin inhibitors, serine protease inhibitors, patatin, cystatin, papain inhibitors; ribosome-
inactivating proteins (RIP), such as ricin, maize-RIP, abrin, luffin, saporin or bryodin; steroid metabolism enzymes, such as 3-hydroxysteroidoxidase, ecdysteroid-UDP-glycosyl-transferase, cholesterol oxidases, ecdysone inhibitors, HMG-COA-reductase, ion channel blockers, such as blockers of sodium or calcium channels, juvenile hormone esterase, diuretic hormone receptors, stilbene synthase, bibenzyl synthase, chitinases and glucanases. Further, in the context of the present invention there are to be understood by ^-endotoxins, for example Cry1Ab, Cry1Ac, Cry1F, Cry1Fa2, Cry2Ab, Cry3A, Cry3Bb1 or Cry9C, or vegetative insecticidal proteins (Vip), for example Vip1, Vip2, Vip3 or Vip3A, expressly also hybrid toxins, truncated toxins and modified toxins. Hybrid toxins are produced recombinantly by a new combination of different domains of those proteins (see, for example, WO 02/15701). Truncated toxins, for example a truncated Cry1Ab, are known. In the case of modified toxins, one or more amino acids of the naturally occurring toxin are replaced. In such amino acid replacements, preferably non-naturally present protease recognition sequences are inserted into the toxin, such as, for example, in the case of Cry3A055, a cathepsin-G- recognition sequence is inserted into a Cry3A toxin (see WO03/018810). More examples of such toxins or transgenic plants capable of synthesising such toxins are disclosed, for example, in EP-A-0374753, WO93/07278, WO95/34656, EP-A-0427529, EP-A-451878 and WO03/052073. The processes for the preparation of such transgenic plants are generally known to the person skilled in the art and are described, for example, in the publications mentioned above. CryI-type deoxyribonucleic acids and their preparation are known, for example, from WO 95/34656, EP-A-0367 474, EP-A-0401979 and WO 90/13651. The toxin contained in the transgenic plants imparts to the plants tolerance to harmful insects. Such insects can occur in any taxonomic group of insects, but are especially commonly found in the beetles (Coleoptera), two-winged insects (Diptera) and butterflies (Lepidoptera). Transgenic plants containing one or more genes that code for an insecticidal resistance and express one or more toxins are known and some of them are commercially available. Examples of such plants are: YieldGard ^ (maize variety that expresses a Cry1Ab toxin); YieldGard Rootworm ^ (maize variety that expresses a Cry3Bb1 toxin); YieldGard Plus ^ (maize variety that expresses a Cry1Ab and a Cry3Bb1 toxin); Starlink ^ (maize variety that expresses a Cry9C toxin); Herculex I ^ (maize variety that expresses a Cry1Fa2 toxin and the enzyme phosphinothricine N-acetyltransferase (PAT) to achieve tolerance to the herbicide glufosinate ammonium); NuCOTN 33B ^ (cotton variety that expresses a Cry1Ac toxin); Bollgard I ^ (cotton variety that expresses a Cry1Ac toxin); Bollgard II® (cotton variety that expresses a Cry1Ac and a Cry2Ab toxin); VipCot ^ (cotton variety that expresses a Vip3A and a Cry1Ab toxin); NewLeaf ^ (potato variety that expresses a Cry3A toxin); NatureGard ^, Agrisure® GT
Advantage (GA21 glyphosate-tolerant trait), Agrisure® CB Advantage (Bt11 corn borer (CB) trait) and Protecta ^. Further examples of such transgenic crops are: 1. Bt11 Maize from Syngenta Seeds SAS, Chemin de l'Hobit 27, F-31 790 St. Sauveur, France, registration number C/FR/96/05/10. Genetically modified Zea mays which has been rendered resistant to attack by the European corn borer (Ostrinia nubilalis and Sesamia nonagrioides) by transgenic expression of a truncated Cry1Ab toxin. Bt11 maize also transgenically expresses the enzyme PAT to achieve tolerance to the herbicide glufosinate ammonium. 2. Bt176 Maize from Syngenta Seeds SAS, Chemin de l'Hobit 27, F-31790 St. Sauveur, France, registration number C/FR/96/05/10. Genetically modified Zea mays which has been rendered resistant to attack by the European corn borer (Ostrinia nubilalis and Sesamia nonagrioides) by transgenic expression of a Cry1Ab toxin. Bt176 maize also transgenically expresses the enzyme PAT to achieve tolerance to the herbicide glufosinate ammonium. 3. MIR604 Maize from Syngenta Seeds SAS, Chemin de l'Hobit 27, F-31790 St. Sauveur, France, registration number C/FR/96/05/10. Maize which has been rendered insect-resistant by transgenic expression of a modified Cry3A toxin. This toxin is Cry3A055 modified by insertion of a cathepsin-G- protease recognition sequence. The preparation of such transgenic maize plants is described in WO 03/018810. 4. MON 863 Maize from Monsanto Europe S.A. 270-272 Avenue de Tervuren, B-1150 Brussels, Belgium, registration number C/DE/02/9. MON 863 expresses a Cry3Bb1 toxin and has resistance to certain Coleoptera insects. 5. IPC 531 Cotton from Monsanto Europe S.A. 270-272 Avenue de Tervuren, B-1150 Brussels, Belgium, registration number C/ES/96/02. 6. 1507 Maize from Pioneer Overseas Corporation, Avenue Tedesco, 7 B-1160 Brussels, Belgium, registration number C/NL/00/10. Genetically modified maize for the expression of the protein Cry1F for achieving resistance to certain Lepidoptera insects and of the PAT protein for achieving tolerance to the herbicide glufosinate ammonium. 7. NK603 × MON 810 Maize from Monsanto Europe S.A. 270-272 Avenue de Tervuren, B-1150 Brussels, Belgium, registration number C/GB/02/M3/03. Consists of conventionally bred hybrid maize varieties by crossing the genetically modified varieties NK603 and MON 810. NK603 × MON 810 Maize transgenically expresses the protein CP4 EPSPS, obtained from Agrobacterium sp. strain CP4, which imparts tolerance to the herbicide Roundup® (contains glyphosate), and also a Cry1Ab toxin obtained from Bacillus thuringiensis subsp. kurstaki which brings about tolerance to certain Lepidoptera, include the European corn borer. The term “locus” as used herein means fields in or on which plants are growing, or where seeds of cultivated plants are sown, or where seed will be placed into the soil. It includes soil, seeds, and seedlings, as well as established vegetation. The term “plants” refers to all physical parts of a plant, including seeds, seedlings, saplings, roots, tubers, stems, stalks, foliage, and fruits.
The term “plant propagation material” is understood to denote generative parts of the plant, such as seeds, which can be used for the multiplication of the latter, and vegetative material, such as cuttings or tubers, for example potatoes. There may be mentioned for example seeds (in the strict sense), roots, fruits, tubers, bulbs, rhizomes and parts of plants. Germinated plants and young plants which are to be transplanted after germination or after emergence from the soil, may also be mentioned. These young plants may be protected before transplantation by a total or partial treatment by immersion. Preferably “plant propagation material” is understood to denote seeds. Pesticidal agents referred to herein using their common name are known, for example, from "The Pesticide Manual", 19th Ed., British Crop Protection Council 2021. The compounds of formula (I) as defined in the present invention may be used in unmodified form or, preferably, together with the adjuvants conventionally employed in the art of formulation. To this end they may be conveniently formulated in known manner to emulsifiable concentrates, coatable pastes, directly sprayable or dilutable solutions or suspensions, dilute emulsions, wettable powders, soluble powders, dusts, granulates, and also encapsulations e.g. in polymeric substances. As with the type of the compositions, the methods of application, such as spraying, atomising, dusting, scattering, coating or pouring, are chosen in accordance with the intended objectives and the prevailing circumstances. The compositions may also contain further adjuvants such as stabilizers, antifoams, viscosity regulators, binders or tackifiers as well as fertilizers, micronutrient donors or other formulations for obtaining special effects. Suitable carriers and/or adjuvants, e.g. for agricultural use, can be solid or liquid and are substances useful in formulation technology, e.g. natural or regenerated mineral substances, solvents, dispersants, wetting agents, tackifiers, thickeners, binders or fertilizers. Such carriers are for example described in WO 97/33890. Suspension concentrates are aqueous formulations in which finely divided solid particles of the active compound are suspended. Such formulations include anti-settling agents and dispersing agents and may further include a wetting agent to enhance activity as well an anti-foam and a crystal growth inhibitor. In use, these concentrates are diluted in water and normally applied as a spray to the area to be treated. The amount of active ingredient may range from 0.5% to 95% of the concentrate. Wettable powders are in the form of finely divided particles which disperse readily in water or other liquid carriers. The particles contain the active ingredient retained in a solid matrix. Typical solid matrices include fuller’s earth, kaolin clays, silicas and other readily wet organic or inorganic solids. Wettable powders normally contain from 5% to 95% of the active ingredient plus a small amount of wetting, dispersing or emulsifying agent. Emulsifiable concentrates are homogeneous liquid compositions dispersible in water or other liquid and may consist entirely of the active compound with a liquid or solid emulsifying agent, or may also contain a liquid carrier, such as xylene, heavy aromatic naphthas, isophorone and other non-volatile organic
solvents. In use, these concentrates are dispersed in water or other liquid and normally applied as a spray to the area to be treated. The amount of active ingredient may range from 0.5% to 95% of the concentrate. Granular formulations include both extrudates and relatively coarse particles and are usually applied without dilution to the area in which treatment is required. Typical carriers for granular formulations include sand, fuller’s earth, attapulgite clay, bentonite clays, montmorillonite clay, vermiculite, perlite, calcium carbonate, brick, pumice, pyrophyllite, kaolin, dolomite, plaster, wood flour, ground corn cobs, ground peanut hulls, sugars, sodium chloride, sodium sulphate, sodium silicate, sodium borate, magnesia, mica, iron oxide, zinc oxide, titanium oxide, antimony oxide, cryolite, gypsum, diatomaceous earth, calcium sulphate and other organic or inorganic materials which absorb or which can be coated with the active compound. Granular formulations normally contain 5% to 25% of active ingredients which may include surface-active agents such as heavy aromatic naphthas, kerosene and other petroleum fractions, or vegetable oils; and/or stickers such as dextrins, glue or synthetic resins. Dusts are free-flowing admixtures of the active ingredient with finely divided solids such as talc, clays, flours and other organic and inorganic solids which act as dispersants and carriers. Microcapsules are typically droplets or granules of the active ingredient enclosed in an inert porous shell which allows escape of the enclosed material to the surroundings at controlled rates. Encapsulated droplets are typically 1 to 50 microns in diameter. The enclosed liquid typically constitutes 50 to 95% of the weight of the capsule and may include solvent in addition to the active compound. Encapsulated granules are generally porous granules with porous membranes sealing the granule pore openings, retaining the active species in liquid form inside the granule pores. Granules typically range from 1 millimetre to 1 centimetre and preferably 1 to 2 millimetres in diameter. Granules are formed by extrusion, agglomeration or prilling, or are naturally occurring. Examples of such materials are vermiculite, sintered clay, kaolin, attapulgite clay, sawdust and granular carbon. Shell or membrane materials include natural and synthetic rubbers, cellulosic materials, styrene-butadiene copolymers, polyacrylonitriles, polyacrylates, polyesters, polyamides, polyureas, polyurethanes and starch xanthates. Other useful formulations for agrochemical applications include simple solutions of the active ingredient in a solvent in which it is completely soluble at the desired concentration, such as acetone, alkylated naphthalenes, xylene and other organic solvents. Pressurised sprayers, wherein the active ingredient is dispersed in finely-divided form as a result of vaporisation of a low boiling dispersant solvent carrier, may also be used. Suitable agricultural adjuvants and/or carriers that are useful in formulating the compositions of the invention in the formulation types described above are well known to those skilled in the art. Liquid carriers that can be employed include, for example, water, toluene, xylene, petroleum naphtha, crop oil, acetone, methyl ethyl ketone, cyclohexanone, acetic anhydride, acetonitrile, acetophenone,
amyl acetate, 2-butanone, chlorobenzene, cyclohexane, cyclohexanol, alkyl acetates, diacetonalcohol, 1,2-dichloropropane, diethanolamine, p-diethylbenzene, diethylene glycol, diethylene glycol abietate, diethylene glycol butyl ether, diethylene glycol ethyl ether, diethylene glycol methyl ether, N,N-dimethyl formamide, dimethyl sulfoxide, 1,4-dioxane, dipropylene glycol, dipropylene glycol methyl ether, dipropylene glycol dibenzoate, diproxitol, alkyl pyrrolidinone, ethyl acetate, 2-ethyl hexanol, ethylene carbonate, 1,1,1-trichloroethane, 2-heptanone, alpha pinene, d-limonene, ethylene glycol, ethylene glycol butyl ether, ethylene glycol methyl ether, gamma-butyrolactone, glycerol, glycerol diacetate, glycerol monoacetate, glycerol triacetate, hexadecane, hexylene glycol, isoamyl acetate, isobornyl acetate, isooctane, isophorone, isopropyl benzene, isopropyl myristate, lactic acid, laurylamine, mesityl oxide, methoxy-propanol, methyl isoamyl ketone, methyl isobutyl ketone, methyl laurate, methyl octanoate, methyl oleate, methylene chloride, m-xylene, n-hexane, n-octylamine, octadecanoic acid, octyl amine acetate, oleic acid, oleylamine, o-xylene, phenol, polyethylene glycol (PEG400), propionic acid, propylene glycol, propylene glycol monomethyl ether, p-xylene, toluene, triethyl phosphate, triethylene glycol, xylene sulfonic acid, paraffin, mineral oil, trichloroethylene, perchloroethylene, ethyl acetate, amyl acetate, butyl acetate, methanol, ethanol, isopropanol, and higher molecular weight alcohols such as amyl alcohol, tetrahydrofurfuryl alcohol, hexanol, octanol, etc., ethylene glycol, propylene glycol, glycerine and N-methyl-2-pyrrolidinone. Water is generally the carrier of choice for the dilution of concentrates. Suitable solid carriers include, for example, talc, titanium dioxide, pyrophyllite clay, silica, attapulgite clay, kieselguhr, chalk, diatomaxeous earth, lime, calcium carbonate, bentonite clay, fuller’s earth, cotton seed hulls, wheat flour, soybean flour, pumice, wood flour, walnut shell flour and lignin. A broad range of surface-active agents are advantageously employed in both said liquid and solid compositions, especially those designed to be diluted with carrier before application. These agents, when used, normally comprise from 0.1% to 15% by weight of the formulation. They can be anionic, cationic, non-ionic or polymeric in character and can be employed as emulsifying agents, wetting agents, suspending agents or for other purposes. Typical surface active agents include salts of alkyl sulfates, such as diethanolammonium lauryl sulphate; alkylarylsulfonate salts, such as calcium dodecylbenzenesulfonate; alkylphenol-alkylene oxide addition products, such as nonylphenol-C.sub.18 ethoxylate; alcohol-alkylene oxide addition products, such as tridecyl alcohol-C.sub. 16 ethoxylate; soaps, such as sodium stearate; alkylnaphthalenesulfonate salts, such as sodium dibutylnaphthalenesulfonate; dialkyl esters of sulfosuccinate salts, such as sodium di(2-ethylhexyl) sulfosuccinate; sorbitol esters, such as sorbitol oleate; quaternary amines, such as lauryl trimethylammonium chloride; polyethylene glycol esters of fatty acids, such as polyethylene glycol stearate; block copolymers of ethylene oxide and propylene oxide; and salts of mono and dialkyl phosphate esters. Other adjuvants commonly utilized in agricultural compositions include crystallisation inhibitors, viscosity modifiers, suspending agents, spray droplet modifiers, pigments, antioxidants, foaming agents, anti- foaming agents, light-blocking agents, compatibilizing agents, antifoam agents, sequestering agents,
neutralising agents and buffers, corrosion inhibitors, dyes, odorants, spreading agents, penetration aids, micronutrients, emollients, lubricants and sticking agents. In addition, further, other biocidally active ingredients or compositions may be combined with the compositions of the invention and used in the methods of the invention and applied simultaneously or sequentially with the compositions of the invention. When applied simultaneously, these further active ingredients may be formulated together with the compositions of the invention or mixed in, for example, the spray tank. These further biocidally active ingredients may be fungicides, herbicides, insecticides, bactericides, acaricides, nematicides, plant growth regulators, and/or biologicals. The following combinations of a compound of formula I with another active substance in a weight ratio of 1:1 are preferred (where the abbreviation “TX” means “one compound selected from the compounds defined in the Tables 1.1 to 1.132 and Table A): an adjuvant selected from the group of substances consisting of petroleum oils (alternative name) (628) + TX; abamectin + TX, acequinocyl + TX, acetamiprid + TX, acetoprole + TX, acrinathrin + TX, acynonapyr + TX, afidopyropen + TX, afoxolaner + TX, alanycarb + TX, allethrin + TX, alpha-cypermethrin + TX, alphamethrin + TX, amidoflumet + TX, aminocarb + TX, azocyclotin + TX, bensultap + TX, benzoximate + TX, benzpyrimoxan + TX, betacyfluthrin + TX, beta-cypermethrin + TX, bifenazate + TX, bifenthrin + TX, binapacryl + TX, bioallethrin + TX, S-bioallethrin + TX, bioresmethrin + TX, bistrifluron + TX, broflanilide + TX, brofluthrinate + TX, bromophos-ethyl + TX, buprofezine + TX, butocarboxim + TX, cadusafos + TX, carbaryl + TX, carbosulfan + TX, cartap + TX, CAS number: 1632218-00-8 + TX, CAS number: 1808115-49-2 + TX, CAS number: 2032403-97-5 + TX, CAS number: 2044701-44-0 + TX, CAS number: 2128706-05-6 + TX, CAS number: 2095470-94-1 + TX, CAS number: 2377084-09-6 + TX, CAS number: 1445683-71-5 + TX, CAS number: 2408220-94-8 + TX, CAS number: 2408220-91-5 + TX, CAS number: 1365070-72-9 + TX, CAS number: 2171099-09-3 + TX, CAS number: 2396747-83-2 + TX, CAS number: 2133042-31-4 + TX, CAS number: 2133042-44-9 + TX, CAS number: 1445684-82-1 + TX, CAS number: 1445684-82-1 + TX, CAS number: 1922957-45-6 + TX, CAS number: 1922957-46- 7 + TX, CAS number: 1922957-47-8 + TX, CAS number: 1922957-48-9 + TX, CAS number: 2415706- 16-8 + TX, CAS number: 1594624-87-9 + TX, CAS number: 1594637-65-6 + TX, CAS number: 1594626-19-3 + TX, CAS number: 1990457-52-7 + TX, CAS number: 1990457-55-0 + TX, CAS number: 1990457-57-2 + TX, CAS number: 1990457-77-6 + TX, CAS number: 1990457-66-3 + TX, CAS number: 1990457-85-6 + TX, CAS number: 2220132-55-6 + TX, CAS number: 1255091-74-7 + TX, CAS number: 2719848-60-7 + TX, CAS number: 1956329-03-5 + TX, chlorantraniliprole + TX, chlordane + TX, chlorfenapyr + TX, chloroprallethrin + TX, chromafenozide + TX, clenpirin + TX, cloethocarb + TX, clothianidin + TX, 2-chlorophenyl N-methylcarbamate (CPMC) + TX, cyanofenphos + TX, cyantraniliprole + TX, cyclaniliprole + TX, cyclobutrifluram + TX, cycloprothrin + TX, cycloxaprid + TX, cyenopyrafen + TX, cyetpyrafen (or etpyrafen) + TX, cyflumetofen + TX, cyfluthrin + TX, cyhalodiamide + TX, cyhalothrin + TX, cypermethrin + TX, cyphenothrin + TX, cyproflanilide + TX, cyromazine + TX, deltamethrin + TX, diafenthiuron + TX, dialifos + TX, dibrom + TX, dicloromezotiaz + TX, diflovidazine + TX, diflubenzuron + TX, dimpropyridaz + TX, dinactin + TX, dinocap + TX, dinotefuran + TX, dioxabenzofos + TX, emamectin (or emamectin benzoate) + TX, empenthrin + TX, epsilon -
momfluorothrin + TX, epsilon-metofluthrin + TX, esfenvalerate + TX, ethion + TX, ethiprole + TX, etofenprox + TX, etoxazole + TX, famphur + TX, fenazaquin + TX, fenfluthrin + TX, , fenmezoditiaz + TX, fenitrothion + TX, fenobucarb + TX, fenothiocarb + TX, fenoxycarb + TX, fenpropathrin + TX, fenpyroximate + TX, fensulfothion + TX, fenthion + TX, fentinacetate + TX, fenvalerate + TX, fipronil + TX, flometoquin + TX, flonicamid + TX, fluacrypyrim + TX, fluazaindolizine + TX, fluazuron + TX, flubendiamide + TX, flubenzimine + TX, fluchlordiniliprole + TX, flucitrinate + TX, flucycloxuron + TX, flucythrinate + TX, fluensulfone + TX, flufenerim + TX, flufenprox + TX, flufiprole + TX, fluhexafon + TX, flumethrin + TX, fluopyram + TX, flupentiofenox + TX, flupyradifurone + TX, flupyroxystrobin + TX, flupyrimin + TX, fluralaner + TX, fluvalinate + TX, fluxametamide + TX, fosthiazate + TX, gamma- cyhalothrin + TX, guadipyr + TX, halofenozide + TX, halfenprox + TX, heptafluthrin + TX, hexythiazox + TX, hydramethylnon + TX, imicyafos + TX, imidacloprid + TX, imiprothrin + TX, indazapyroxamet + TX, indoxacarb + TX, iodomethane + TX, iprodione + TX, isocycloseram + TX, isothioate + TX, ivermectin + TX, kappa-bifenthrin + TX, kappa-tefluthrin + TX, lambda-Cyhalothrin + TX, ledprona + TX, lepimectin + TX, lotilaner + TX, lufenuron + TX, metaflumizone + TX, metaldehyde + TX, metam + TX, methomyl + TX, methoxyfenozide + TX, metofluthrin + TX, metolcarb + TX, mexacarbate + TX, milbemectin + TX, momfluorothrin + TX, niclosamide + TX, nicofluprole + TX; nitenpyram + TX, nithiazine + TX, omethoate + TX, oxamyl + TX, oxazosulfyl + TX, parathion-ethyl + TX, permethrin + TX, phenothrin + TX, phosphocarb + TX, piperonylbutoxide + TX, pirimicarb + TX, pirimiphos-ethyl + TX, pirimiphos-methyl + TX, Polyhedrosis virus + TX, prallethrin + TX, profenofos + TX, profluthrin + TX, propargite + TX, propetamphos + TX, propoxur + TX, prothiophos + TX, protrifenbute + TX, pyflubumide + TX, pymetrozine + TX, pyraclofos + TX, pyrafluprole + TX, pyridaben + TX, pyridalyl + TX, pyrifluquinazon + TX, pyrimidifen + TX, pyriminostrobin + TX, pyriprole + TX, pyriproxyfen + TX, resmethrin + TX, sarolaner + TX, selamectin + TX, silafluofen + TX, spinetoram + TX, spinosad + TX, spirobudifen + TX; spirodiclofen + TX, spiromesifen + TX, spiropidion + TX, spirotetramat + TX, spidoxamat + TX, sulfoxaflor + TX, tebufenozide + TX, tebufenpyrad + TX, tebupirimiphos + TX, tefluthrin + TX, temephos + TX, tetrachlorantraniliprole + TX, tetradiphon + TX, tetramethrin + TX, tetramethylfluthrin + TX, tetranactin + TX, tetraniliprole + TX, theta-cypermethrin + TX, thiacloprid + TX, thiamethoxam + TX, thiocyclam + TX, thiodicarb + TX, thiofanox + TX, thiometon + TX, thiosultap + TX, tigolaner + TX, tiorantraniliprole + TX; tioxazafen + TX, tolfenpyrad + TX, toxaphene + TX, tralomethrin + TX, transfluthrin + TX, triazamate + TX, triazophos + TX, trichlorfon + TX, trichloronate + TX, trichlorphon + TX, trifluenfuronate + TX, triflumezopyrim + TX, tyclopyrazoflor + TX, zeta-cypermethrin + TX, Extract of seaweed and fermentation product derived from melasse + TX, Extract of seaweed and fermentation product derived from melasse comprising urea + TX, amino acids + TX, potassium and molybdenum and EDTA-chelated manganese + TX, Extract of seaweed and fermented plant products + TX, Extract of seaweed and fermented plant products comprising phytohormones + TX, vitamins + TX, EDTA- chelated copper + TX, zinc + TX, and iron + TX, azadirachtin + TX, Bacillus aizawai + TX, Bacillus chitinosporus AQ746 (NRRL Accession No B-21618) + TX, Bacillus firmus + TX, Bacillus kurstaki + TX, Bacillus mycoides AQ726 (NRRL Accession No. B-21664) + TX, Bacillus pumilus (NRRL Accession No B-30087) + TX, Bacillus pumilus AQ717 (NRRL Accession No. B-21662) + TX, Bacillus sp. AQ178 (ATCC Accession No.53522) + TX, Bacillus sp. AQ175 (ATCC Accession No.55608) + TX, Bacillus sp. AQ177 (ATCC Accession No.55609) + TX, Bacillus subtilis unspecified + TX, Bacillus subtilis AQ153 (ATCC Accession No.55614) + TX, Bacillus subtilis AQ30002 (NRRL Accession No. B-50421) + TX,
Bacillus subtilis AQ30004 (NRRL Accession No. B- 50455) + TX, Bacillus subtilis AQ713 (NRRL Accession No. B-21661) + TX, Bacillus subtilis AQ743 (NRRL Accession No. B-21665) + TX, Bacillus thuringiensis AQ52 (NRRL Accession No. B-21619) + TX, Bacillus thuringiensis BD#32 (NRRL Accession No B-21530) + TX, Bacillus thuringiensis subspec. kurstaki BMP 123 + TX, Beauveria bassiana + TX, D-limonene + TX, Granulovirus + TX, Harpin + TX, Helicoverpa armigera Nucleopolyhedrovirus + TX, Helicoverpa zea Nucleopolyhedrovirus + TX, Heliothis virescens Nucleopolyhedrovirus + TX, Heliothis punctigera Nucleopolyhedrovirus + TX, Metarhizium spp. + TX, Muscodor albus 620 (NRRL Accession No.30547) + TX, Muscodor roseus A3-5 (NRRL Accession No. 30548) + TX, Neem tree based products + TX, Paecilomyces fumosoroseus + TX, Paecilomyces lilacinus + TX, Pasteuria nishizawae + TX, Pasteuria penetrans + TX, Pasteuria ramosa + TX, Pasteuria thornei + TX, Pasteuria usgae + TX, P-cymene + TX, Plutella xylostella Granulosis virus + TX, Plutella xylostella Nucleopolyhedrovirus + TX, Polyhedrosis virus + TX, pyrethrum + TX, QRD 420 (a terpenoid blend) + TX, QRD 452 (a terpenoid blend) + TX, QRD 460 (a terpenoid blend) + TX, Quillaja saponaria + TX, Rhodococcus globerulus AQ719 (NRRL Accession No B-21663) + TX, Spodoptera frugiperda Nucleopolyhedrovirus + TX, Streptomyces galbus (NRRL Accession No.30232) + TX, Streptomyces sp. (NRRL Accession No. B-30145) + TX, Terpenoid blend + TX, and Verticillium spp. + TX; an algicide selected from the group of substances consisting of bethoxazin [CCN] + TX, copper dioctanoate (IUPAC name) (170) + TX, copper sulfate (172) + TX, cybutryne [CCN] + TX, dichlone (1052) + TX, dichlorophen (232) + TX, endothal (295) + TX, fentin (347) + TX, hydrated lime [CCN] + TX, nabam (566) + TX, quinoclamine (714) + TX, quinonamid (1379) + TX, simazine (730) + TX, triphenyltin acetate (IUPAC name) (347) + TX, and triphenyltin hydroxide (IUPAC name) (347) + TX; an anthelmintic selected from the group of substances consisting of abamectin (1) + TX, crufomate (1011) + TX, cyclobutrifluram + TX, doramectin (alternative name) [CCN] + TX, emamectin (291) + TX, emamectin benzoate (291) + TX, eprinomectin (alternative name) [CCN] + TX, ivermectin (alternative name) [CCN] + TX, milbemycin oxime (alternative name) [CCN] + TX, moxidectin (alternative name) [CCN] + TX, piperazine [CCN] + TX, selamectin (alternative name) [CCN] + TX, spinosad (737) + TX, and thiophanate (1435) + TX; an avicide selected from the group of substances consisting of chloralose (127) + TX, endrin (1122) + TX, fenthion (346) + TX, pyridin-4-amine (IUPAC name) (23) + TX, and strychnine (745) + TX; a bactericide selected from the group of substances consisting of 1-hydroxy-1H-pyridine-2-thione (IUPAC name) (1222) + TX, 4-(quinoxalin-2-ylamino)benzenesulfonamide (IUPAC name) (748) + TX, 8-hydroxyquinoline sulfate (446) + TX, bronopol (97) + TX, copper dioctanoate (IUPAC name) (170) + TX, copper hydroxide (IUPAC name) (169) + TX, cresol [CCN] + TX, dichlorophen (232) + TX, dipyrithione (1105) + TX, dodicin (1112) + TX, fenaminosulf (1144) + TX, formaldehyde (404) + TX, hydrargaphen (alternative name) [CCN] + TX, kasugamycin (483) + TX, kasugamycin hydrochloride hydrate (483) + TX, nickel bis(dimethyldithiocarbamate) (IUPAC name) (1308) + TX, nitrapyrin (580) + TX, octhilinone (590) + TX, oxolinic acid (606) + TX, oxytetracycline (611) + TX, potassium hydroxyquinoline sulfate (446) + TX, probenazole (658) + TX, streptomycin (744) + TX, streptomycin sesquisulfate (744) + TX, tecloftalam (766) + TX, and thiomersal (alternative name) [CCN] + TX; a biological agent selected from the group of substances consisting of Adoxophyes orana GV (alternative name) (12) + TX, Agrobacterium radiobacter (alternative name) (13) + TX, Amblyseius spp. (alternative name) (19) + TX, Anagrapha falcifera NPV (alternative name) (28) + TX, Anagrus
atomus (alternative name) (29) + TX, Aphelinus abdominalis (alternative name) (33) + TX, Aphidius colemani (alternative name) (34) + TX, Aphidoletes aphidimyza (alternative name) (35) + TX, Autographa californica NPV (alternative name) (38) + TX, Bacillus firmus (alternative name) (48) + TX, Bacillus sphaericus Neide (scientific name) (49) + TX, Bacillus thuringiensis Berliner (scientific name) (51) + TX, Bacillus thuringiensis subsp. aizawai (scientific name) (51) + TX, Bacillus thuringiensis subsp. israelensis (scientific name) (51) + TX, Bacillus thuringiensis subsp. japonensis (scientific name) (51) + TX, Bacillus thuringiensis subsp. kurstaki (scientific name) (51) + TX, Bacillus thuringiensis subsp. tenebrionis (scientific name) (51) + TX, Beauveria bassiana (alternative name) (53) + TX, Beauveria brongniartii (alternative name) (54) + TX, Chrysoperla carnea (alternative name) (151) + TX, Cryptolaemus montrouzieri (alternative name) (178) + TX, Cydia pomonella GV (alternative name) (191) + TX, Dacnusa sibirica (alternative name) (212) + TX, Diglyphus isaea (alternative name) (254) + TX, Encarsia formosa (scientific name) (293) + TX, Eretmocerus eremicus (alternative name) (300) + TX, Helicoverpa zea NPV (alternative name) (431) + TX, Heterorhabditis bacteriophora and H. megidis (alternative name) (433) + TX, Hippodamia convergens (alternative name) (442) + TX, Leptomastix dactylopii (alternative name) (488) + TX, Macrolophus caliginosus (alternative name) (491) + TX, Mamestra brassicae NPV (alternative name) (494) + TX, Metaphycus helvolus (alternative name) (522) + TX, Metarhizium anisopliae var. acridum (scientific name) (523) + TX, Metarhizium anisopliae var. anisopliae (scientific name) (523) + TX, Neodiprion sertifer NPV and N. lecontei NPV (alternative name) (575) + TX, Orius spp. (alternative name) (596) + TX, Paecilomyces fumosoroseus (alternative name) (613) + TX, Phytoseiulus persimilis (alternative name) (644) + TX, Spodoptera exigua multicapsid nuclear polyhedrosis virus (scientific name) (741) + TX, Steinernema bibionis (alternative name) (742) + TX, Steinernema carpocapsae (alternative name) (742) + TX, Steinernema feltiae (alternative name) (742) + TX, Steinernema glaseri (alternative name) (742) + TX, Steinernema riobrave (alternative name) (742) + TX, Steinernema riobravis (alternative name) (742) + TX, Steinernema scapterisci (alternative name) (742) + TX, Steinernema spp. (alternative name) (742) + TX, Trichogramma spp. (alternative name) (826) + TX, Typhlodromus occidentalis (alternative name) (844) + TX, and Verticillium lecanii (alternative name) (848) + TX; a soil sterilant selected from the group of substances consisting of iodomethane (IUPAC name) (542) + TX, and methyl bromide (537) + TX; a chemosterilant selected from the group of substances consisting of apholate [CCN] + TX, bisazir (alternative name) [CCN] + TX, busulfan (alternative name) [CCN] + TX, diflubenzuron (250) + TX, dimatif (alternative name) [CCN] + TX, hemel [CCN] + TX, hempa [CCN] + TX, metepa [CCN] + TX, methiotepa [CCN] + TX, methyl apholate [CCN] + TX, morzid [CCN] + TX, penfluron (alternative name) [CCN] + TX, tepa [CCN] + TX, thiohempa (alternative name) [CCN] + TX, thiotepa (alternative name) [CCN] + TX, tretamine (alternative name) [CCN] + TX, and uredepa (alternative name) [CCN] + TX; an insect pheromone selected from the group of substances consisting of (E)-dec-5-en-1-yl acetate with (E)-dec-5-en-1-ol (IUPAC name) (222) + TX, (E)-tridec-4-en-1-yl acetate (IUPAC name) (829) + TX, (E)-6-methylhept-2-en-4-ol (IUPAC name) (541) + TX, (E,Z)-tetradeca-4,10-dien-1-yl acetate (IUPAC name) (779) + TX, (Z)-dodec-7-en-1-yl acetate (IUPAC name) (285) + TX, (Z)-hexadec-11-enal (IUPAC name) (436) + TX, (Z)-hexadec-11-en-1-yl acetate (IUPAC name) (437) + TX, (Z)-hexadec- 13-en-11-yn-1-yl acetate (IUPAC name) (438) + TX, (Z)-icos-13-en-10-one (IUPAC name) (448) + TX,
(Z)-tetradec-7-en-1-al (IUPAC name) (782) + TX, (Z)-tetradec-9-en-1-ol (IUPAC name) (783) + TX, (Z)-tetradec-9-en-1-yl acetate (IUPAC name) (784) + TX, (7E,9Z)-dodeca-7,9-dien-1-yl acetate (IUPAC name) (283) + TX, (9Z,11E)-tetradeca-9,11-dien-1-yl acetate (IUPAC name) (780) + TX, (9Z,12E)- tetradeca-9,12-dien-1-yl acetate (IUPAC name) (781) + TX, 14-methyloctadec-1-ene (IUPAC name) (545) + TX, 4-methylnonan-5-ol with 4-methylnonan-5-one (IUPAC name) (544) + TX, alpha- multistriatin (alternative name) [CCN] + TX, brevicomin (alternative name) [CCN] + TX, codlelure (alternative name) [CCN] + TX, codlemone (alternative name) (167) + TX, cuelure (alternative name) (179) + TX, disparlure (277) + TX, dodec-8-en-1-yl acetate (IUPAC name) (286) + TX, dodec-9-en- 1-yl acetate (IUPAC name) (287) + TX, dodeca-8 + TX, 10-dien-1-yl acetate (IUPAC name) (284) + TX, dominicalure (alternative name) [CCN] + TX, ethyl 4-methyloctanoate (IUPAC name) (317) + TX, eugenol (alternative name) [CCN] + TX, frontalin (alternative name) [CCN] + TX, Gossyplure® (alternative name; 1:1 mixture of the (Z,E) and (Z,Z) isomers of hexadeca-7,11-dien-1-yl-acetate) (420) + TX, grandlure (421) + TX, grandlure I (alternative name) (421) + TX, grandlure II (alternative name) (421) + TX, grandlure III (alternative name) (421) + TX, grandlure IV (alternative name) (421) + TX, hexalure [CCN] + TX, ipsdienol (alternative name) [CCN] + TX, ipsenol (alternative name) [CCN] + TX, japonilure (alternative name) (481) + TX, lineatin (alternative name) [CCN] + TX, litlure (alternative name) [CCN] + TX, looplure (alternative name) [CCN] + TX, medlure [CCN] + TX, megatomoic acid (alternative name) [CCN] + TX, methyl eugenol (alternative name) (540) + TX, muscalure (563) + TX, octadeca-2,13-dien-1-yl acetate (IUPAC name) (588) + TX, octadeca-3,13- dien-1-yl acetate (IUPAC name) (589) + TX, orfralure (alternative name) [CCN] + TX, oryctalure (alternative name) (317) + TX, ostramone (alternative name) [CCN] + TX, siglure [CCN] + TX, sordidin (alternative name) (736) + TX, sulcatol (alternative name) [CCN] + TX, tetradec-11-en-1-yl acetate (IUPAC name) (785) + TX, trimedlure (839) + TX, trimedlure A (alternative name) (839) + TX, trimedlure B1 (alternative name) (839) + TX, trimedlure B2 (alternative name) (839) + TX, trimedlure C (alternative name) (839) TX, and trunc-call (alternative name) [CCN] + TX; an insect repellent selected from the group of substances consisting of 2-(octylthio)ethanol (IUPAC name) (591) + TX, butopyronoxyl (933) + TX, butoxy(polypropylene glycol) (936) + TX, dibutyl adipate (IUPAC name) (1046) + TX, dibutyl phthalate (1047) + TX, dibutyl succinate (IUPAC name) (1048) + TX, diethyltoluamide [CCN] + TX, dimethyl carbate [CCN] + TX, dimethyl phthalate [CCN] + TX, ethyl hexanediol (1137) + TX, hexamide [CCN] + TX, methoquin-butyl (1276) + TX, methylneodecanamide [CCN] + TX, oxamate [CCN] + TX, and picaridin [CCN] + TX; a molluscicide selected from the group of substances consisting of bis(tributyltin) oxide (IUPAC name) (913) + TX, bromoacetamide [CCN] + TX, calcium arsenate [CCN] + TX, cloethocarb (999) + TX, copper acetoarsenite [CCN] + TX, copper sulfate (172) + TX, fentin (347) + TX, ferric phosphate (IUPAC name) (352) + TX, metaldehyde (518) + TX, methiocarb (530) + TX, niclosamide (576) + TX, niclosamide-olamine (576) + TX, pentachlorophenol (623) + TX, sodium pentachlorophenoxide (623) + TX, tazimcarb (1412) + TX, thiodicarb (799) + TX, tributyltin oxide (913) + TX, trifenmorph (1454) + TX, trimethacarb (840) + TX, triphenyltin acetate (IUPAC name) (347) + TX, triphenyltin hydroxide (IUPAC name) (347) + TX, and pyriprole [394730-71-3] + TX; a nematicide selected from the group of substances consisting of AKD-3088 (compound code) + TX, 1,2-dibromo-3-chloropropane (IUPAC/Chemical Abstracts name) (1045) + TX, 1,2-dichloropropane (IUPAC/ Chemical Abstracts name) (1062) + TX, 1,2-dichloropropane with 1,3-dichloropropene (IUPAC
name) (1063) + TX, 1,3-dichloropropene (233) + TX, 3,4-dichlorotetrahydrothiophene 1,1-dioxide (IUPAC/Chemical Abstracts name) (1065) + TX, 3-(4-chlorophenyl)-5-methylrhodanine (IUPAC name) (980) + TX, 5-methyl-6-thioxo-1,3,5-thiadiazinan-3-ylacetic acid (IUPAC name) (1286) + TX, 6- isopentenylaminopurine (alternative name) (210) + TX, abamectin (1) + TX, acetoprole [CCN] + TX, alanycarb (15) + TX, aldicarb (16) + TX, aldoxycarb (863) + TX, AZ 60541 (compound code) + TX, benclothiaz [CCN] + TX, benomyl (62) + TX, butylpyridaben (alternative name) + TX, cadusafos (109) + TX, carbofuran (118) + TX, carbon disulfide (945) + TX, carbosulfan (119) + TX, chloropicrin (141) + TX, chlorpyrifos (145) + TX, cloethocarb (999) + TX, cyclobutrifluram + TX, cytokinins (alternative name) (210) + TX, dazomet (216) + TX, DBCP (1045) + TX, DCIP (218) + TX, diamidafos (1044) + TX, dichlofenthion (1051) + TX, dicliphos (alternative name) + TX, dimethoate (262) + TX, doramectin (alternative name) [CCN] + TX, emamectin (291) + TX, emamectin benzoate (291) + TX, eprinomectin (alternative name) [CCN] + TX, ethoprophos (312) + TX, ethylene dibromide (316) + TX, fenamiphos (326) + TX, fenpyrad (alternative name) + TX, fensulfothion (1158) + TX, fosthiazate (408) + TX, fosthietan (1196) + TX, furfural (alternative name) [CCN] + TX, GY-81 (development code) (423) + TX, heterophos [CCN] + TX, iodomethane (IUPAC name) (542) + TX, isamidofos (1230) + TX, isazofos (1231) + TX, ivermectin (alternative name) [CCN] + TX, kinetin (alternative name) (210) + TX, mecarphon (1258) + TX, metam (519) + TX, metam-potassium (alternative name) (519) + TX, metam-sodium (519) + TX, methyl bromide (537) + TX, methyl isothiocyanate (543) + TX, milbemycin oxime (alternative name) [CCN] + TX, moxidectin (alternative name) [CCN] + TX, Myrothecium verrucaria composition (alternative name) (565) + TX, NC-184 (compound code) + TX, oxamyl (602) + TX, phorate (636) + TX, phosphamidon (639) + TX, phosphocarb [CCN] + TX, sebufos (alternative name) + TX, selamectin (alternative name) [CCN] + TX, spinosad (737) + TX, terbam (alternative name) + TX, terbufos (773) + TX, tetrachlorothiophene (IUPAC/ Chemical Abstracts name) (1422) + TX, thiafenox (alternative name) + TX, thionazin (1434) + TX, triazophos (820) + TX, triazuron (alternative name) + TX, xylenols [CCN] + TX, YI-5302 (compound code) + TX, zeatin (alternative name) (210) + TX, fluensulfone [318290-98-1] + TX, and fluopyram + TX; a nitrification inhibitor selected from the group of substances consisting of potassium ethylxanthate [CCN] + TX, and nitrapyrin (580) + TX; a plant activator selected from the group of substances consisting of acibenzolar (6) + TX, acibenzolar- S-methyl (6) + TX, probenazole (658) + TX, and Reynoutria sachalinensis extract (alternative name) (720) + TX; a rodenticide selected from the group of substances consisting of 2-isovalerylindan-1,3-dione (IUPAC name) (1246) + TX, 4-(quinoxalin-2-ylamino)benzenesulfonamide (IUPAC name) (748) + TX, alpha- chlorohydrin [CCN] + TX, aluminium phosphide (640) + TX, antu (880) + TX, arsenous oxide (882) + TX, barium carbonate (891) + TX, bisthiosemi (912) + TX, brodifacoum (89) + TX, bromadiolone (including alpha-bromadiolone) + TX, bromethalin (92) + TX, calcium cyanide (444) + TX, chloralose (127) + TX, chlorophacinone (140) + TX, cholecalciferol (alternative name) (850) + TX, coumachlor (1004) + TX, coumafuryl (1005) + TX, coumatetralyl (175) + TX, crimidine (1009) + TX, difenacoum (246) + TX, difethialone (249) + TX, diphacinone (273) + TX, ergocalciferol (301) + TX, flocoumafen (357) + TX, fluoroacetamide (379) + TX, flupropadine (1183) + TX, flupropadine hydrochloride (1183) + TX, gamma-HCH (430) + TX, HCH (430) + TX, hydrogen cyanide (444) + TX, iodomethane (IUPAC name) (542) + TX, lindane (430) + TX, magnesium phosphide (IUPAC name) (640) + TX,
methyl bromide (537) + TX, norbormide (1318) + TX, phosacetim (1336) + TX, phosphine (IUPAC name) (640) + TX, phosphorus [CCN] + TX, pindone (1341) + TX, potassium arsenite [CCN] + TX, pyrinuron (1371) + TX, scilliroside (1390) + TX, sodium arsenite [CCN] + TX, sodium cyanide (444) + TX, sodium fluoroacetate (735) + TX, strychnine (745) + TX, thallium sulfate [CCN] + TX, warfarin (851) + TX, and zinc phosphide (640) + TX; a synergist selected from the group of substances consisting of 2-(2-butoxyethoxy)ethyl piperonylate (IUPAC name) (934) + TX, 5-(1,3-benzodioxol-5-yl)-3-hexylcyclohex-2-enone (IUPAC name) (903) + TX, farnesol with nerolidol (alternative name) (324) + TX, MB-599 (development code) (498) + TX, MGK 264 (development code) (296) + TX, piperonyl butoxide (649) + TX, piprotal (1343) + TX, propyl isomer (1358) + TX, S421 (development code) (724) + TX, sesamex (1393) + TX, sesasmolin (1394) + TX, and sulfoxide (1406) + TX; an animal repellent selected from the group of substances consisting of anthraquinone (32) + TX, chloralose (127) + TX, copper naphthenate [CCN] + TX, copper oxychloride (171) + TX, diazinon (227) + TX, dicyclopentadiene (chemical name) (1069) + TX, guazatine (422) + TX, guazatine acetates (422) + TX, methiocarb (530) + TX, pyridin-4-amine (IUPAC name) (23) + TX, thiram (804) + TX, trimethacarb (840) + TX, zinc naphthenate [CCN] + TX, and ziram (856) + TX; a virucide selected from the group of substances consisting of imanin (alternative name) [CCN] and ribavirin (alternative name) [CCN] + TX; a wound protectant selected from the group of substances consisting of mercuric oxide (512) + TX, octhilinone (590) + TX, and thiophanate-methyl (802) + TX; a biologically active substance selected from 1,1-bis(4-chloro-phenyl)-2-ethoxyethanol + TX, 2,4- dichlorophenyl benzenesulfonate + TX, 2-fluoro-N-methyl-N-1-naphthylacetamide + TX, 4-chlorophenyl phenyl sulfone + TX, acetoprole + TX, aldoxycarb + TX, amidithion + TX, amidothioate + TX, amiton + TX, amiton hydrogen oxalate + TX, amitraz + TX, aramite + TX, arsenous oxide + TX, azobenzene + TX, azothoate + TX, benomyl + TX, benoxa-fos + TX, benzyl benzoate + TX, bixafen + TX, brofenvalerate + TX, bromo-cyclen + TX, bromophos + TX, bromopropylate + TX, buprofezin + TX, butocarboxim + TX, butoxycarboxim + TX, butylpyridaben + TX, calcium polysulfide + TX, camphechlor + TX, carbanolate + TX, carbophenothion + TX, cymiazole + TX, chino-methionat + TX, chlorbenside + TX, chlordimeform + TX, chlordimeform hydrochloride + TX, chlorfenethol + TX, chlorfenson + TX, chlorfensulfide + TX, chlorobenzilate + TX, chloromebuform + TX, chloromethiuron + TX, chloropropylate + TX, chlorthiophos + TX, cinerin I + TX, cinerin II + TX, cinerins + TX, closantel + TX, coumaphos + TX, crotamiton + TX, crotoxyphos + TX, cufraneb + TX, cyanthoate + TX, DCPM + TX, DDT + TX, demephion + TX, demephion-O + TX, demephion-S + TX, demeton-methyl + TX, demeton- O + TX, demeton-O-methyl + TX, demeton-S + TX, demeton-S-methyl + TX, demeton-S-methylsulfon + TX, dichlofluanid + TX, dichlorvos + TX, dicliphos + TX, dienochlor + TX, dimefox + TX, dinex + TX, dinex-diclexine + TX, dinocap-4 + TX, dinocap-6 + TX, dinocton + TX, dino-penton + TX, dinosulfon + TX, dinoterbon + TX, dioxathion + TX, diphenyl sulfone + TX, disulfiram + TX, DNOC + TX, dofenapyn + TX, doramectin + TX, endothion + TX, eprinomectin + TX, ethoate-methyl + TX, etrimfos + TX, fenazaflor + TX, fenbutatin oxide + TX, fenothiocarb + TX, fenpyrad + TX, fen-pyroximate + TX, fenpyrazamine + TX, fenson + TX, fentrifanil + TX, flubenzimine + TX, flucycloxuron + TX, fluenetil + TX, fluorbenside + TX, FMC 1137 + TX, formetanate + TX, formetanate hydrochloride + TX,
formparanate + TX, gamma-HCH + TX, glyodin + TX, halfenprox + TX, hexadecyl cyclopropanecarboxylate + TX, isocarbophos + TX, jasmolin I + TX, jasmolin II + TX, jodfenphos + TX, lindane + TX, malonoben + TX, mecarbam + TX, mephosfolan + TX, mesulfen + TX, methacrifos + TX, methyl bromide + TX, metolcarb + TX, mexacarbate + TX, milbemycin oxime + TX, mipafox + TX, monocrotophos + TX, morphothion + TX, moxidectin + TX, naled + TX, 4-chloro-2-(2-chloro-2-methyl- propyl)-5-[(6-iodo-3-pyridyl)methoxy]pyridazin-3-one + TX, nifluridide + TX, nikkomycins + TX, nitrilacarb + TX, nitrilacarb 1:1 zinc chloride complex + TX, omethoate + TX, oxydeprofos + TX, oxydisulfoton + TX, pp'-DDT + TX, parathion + TX, permethrin + TX, phenkapton + TX, phosalone + TX, phosfolan + TX, phosphamidon + TX, polychloroterpenes + TX, polynactins + TX, proclonol + TX, promacyl + TX, propoxur + TX, prothidathion + TX, prothoate + TX, pyrethrin I + TX, pyrethrin II + TX, pyrethrins + TX, pyridaphenthion + TX, pyrimitate + TX, quinalphos + TX, quintiofos + TX, R-1492 + TX, phosglycin + TX, rotenone + TX, schradan + TX, sebufos + TX, selamectin + TX, sophamide + TX, SSI- 121 + TX, sulfiram + TX, sulfluramid + TX, sulfotep + TX, sulfur + TX, diflovidazin + TX, tau-fluvalinate + TX, TEPP + TX, terbam + TX, tetradifon + TX, tetrasul + TX, thiafenox + TX, thiocarboxime + TX, thiofanox + TX, thiometon + TX, thioquinox + TX, thuringiensin + TX, triamiphos + TX, triarathene + TX, triazophos + TX, triazuron + TX, trifenofos + TX, trinactin + TX, vamidothion + TX, vaniliprole + TX, bethoxazin + TX, copper dioctanoate + TX, copper sulfate + TX, cybutryne + TX, dichlone + TX, dichlorophen + TX, endothal + TX, fentin + TX, hydrated lime + TX, nabam + TX, quinoclamine + TX, quinonamid + TX, simazine + TX, triphenyltin acetate + TX, triphenyltin hydroxide + TX, crufomate + TX, piperazine + TX, thiophanate + TX, chloralose + TX, fenthion + TX, pyridin-4-amine + TX, strychnine + TX, 1-hydroxy-1H-pyridine-2-thione + TX, 4-(quinoxalin-2-ylamino)benzenesulfonamide + TX, 8- hydroxyquinoline sulfate + TX, bronopol + TX, copper hydroxide + TX, cresol + TX, dipyrithione + TX, dodicin + TX, fenaminosulf + TX, formaldehyde + TX, hydrargaphen + TX, kasugamycin + TX, kasugamycin hydrochloride hydrate + TX, nickel bis(dimethyldithiocarbamate) + TX, nitrapyrin + TX, octhilinone + TX, oxolinic acid + TX, oxytetracycline + TX, potassium hydroxyquinoline sulfate + TX, probenazole + TX, streptomycin + TX, streptomycin sesquisulfate + TX, tecloftalam + TX, thiomersal + TX, Adoxophyes orana GV + TX, Agrobacterium radiobacter + TX, Amblyseius spp. + TX, Anagrapha falcifera NPV + TX, Anagrus atomus + TX, Aphelinus abdominalis + TX, Aphidius colemani + TX, Aphidoletes aphidimyza + TX, Autographa californica NPV + TX, Bacillus sphaericus Neide + TX, Beauveria brongniartii + TX, Chrysoperla carnea + TX, Cryptolaemus montrouzieri + TX, Cydia pomonella GV + TX, Dacnusa sibirica + TX, Diglyphus isaea + TX, Encarsia formosa + TX, Eretmocerus eremicus + TX, Heterorhabditis bacteriophora and H. megidis + TX, Hippodamia convergens + TX, Leptomastix dactylopii + TX, Macrolophus caliginosus + TX, Mamestra brassicae NPV + TX, Metaphycus helvolus + TX, Metarhizium anisopliae var. acridum + TX, Metarhizium anisopliae var. anisopliae + TX, Neodiprion sertifer NPV and N. lecontei NPV + TX, Orius spp. + TX, Paecilomyces fumosoroseus + TX, Phytoseiulus persimilis + TX, Steinernema bibionis + TX, Steinernema carpocapsae + TX, Steinernema feltiae + TX, Steinernema glaseri + TX, Steinernema riobrave + TX, Steinernema riobravis + TX, Steinernema scapterisci + TX, Steinernema spp. + TX, Trichogramma spp. + TX, Typhlodromus occidentalis + TX , Verticillium lecanii + TX, apholate + TX, bisazir + TX, busulfan + TX, dimatif + TX, hemel + TX, hempa + TX, metepa + TX, methiotepa + TX, methyl apholate + TX, morzid + TX, penfluron + TX, tepa + TX, thiohempa + TX, thiotepa + TX, tretamine + TX, uredepa + TX, (E)-dec-5-en-1-yl acetate with (E)-dec-5-en-1-ol + TX, (E)-tridec-4-en-1-yl acetate + TX, (E)-6-
methylhept-2-en-4-ol + TX, (E,Z)-tetradeca-4,10-dien-1-yl acetate + TX, (Z)-dodec-7-en-1-yl acetate + TX, (Z)-hexadec-11-enal + TX, (Z)-hexadec-11-en-1-yl acetate + TX, (Z)-hexadec-13-en-11-yn-1-yl acetate + TX, (Z)-icos-13-en-10-one + TX, (Z)-tetradec-7-en-1-al + TX, (Z)-tetradec-9-en-1-ol + TX, (Z)- tetradec-9-en-1-yl acetate + TX, (7E,9Z)-dodeca-7,9-dien-1-yl acetate + TX, (9Z,11E)-tetradeca-9,11- dien-1-yl acetate + TX, (9Z,12E)-tetradeca-9,12-dien-1-yl acetate + TX, 14-methyloctadec-1-ene + TX, 4-methylnonan-5-ol with 4-methylnonan-5-one + TX, alpha-multistriatin + TX, brevicomin + TX, codlelure + TX, codlemone + TX, cuelure + TX, disparlure + TX, dodec-8-en-1-yl acetate + TX, dodec-9-en-1-yl acetate + TX, dodeca-8 + TX, 10-dien-1-yl acetate + TX, dominicalure + TX, ethyl 4-methyloctanoate + TX, eugenol + TX, frontalin + TX, grandlure + TX, grandlure I + TX, grandlure II + TX, grandlure III + TX, grandlure IV + TX, hexalure + TX, ipsdienol + TX, ipsenol + TX, japonilure + TX, lineatin + TX, litlure + TX, looplure + TX, medlure + TX, megatomoic acid + TX, methyl eugenol + TX, muscalure + TX, octadeca-2,13-dien-1-yl acetate + TX, octadeca-3,13-dien-1-yl acetate + TX, orfralure + TX, oryctalure + TX, ostramone + TX, siglure + TX, sordidin + TX, sulcatol + TX, tetradec-11-en-1-yl acetate + TX, trimedlure + TX, trimedlure A + TX, trimedlure B1 + TX, trimedlure B2 + TX, trimedlure C + TX, trunc-call + TX, 2-(octylthio)-ethanol + TX, butopyronoxyl + TX, butoxy(polypropylene glycol) + TX, dibutyl adipate + TX, dibutyl phthalate + TX, dibutyl succinate + TX, diethyltoluamide + TX, dimethyl carbate + TX, dimethyl phthalate + TX, ethyl hexanediol + TX, hexamide + TX, methoquin-butyl + TX, methylneodecanamide + TX, oxamate + TX, picaridin + TX, 1-dichloro-1-nitroethane + TX, 1,1-dichloro- 2,2-bis(4-ethylphenyl)-ethane + TX, 1,2-dichloropropane with 1,3-dichloropropene + TX, 1-bromo-2- chloroethane + TX, 2,2,2-trichloro-1-(3,4-dichloro-phenyl)ethyl acetate + TX, 2,2-dichlorovinyl 2- ethylsulfinylethyl methyl phosphate + TX, 2-(1,3-dithiolan-2-yl)phenyl dimethylcarbamate + TX, 2-(2- butoxyethoxy)ethyl thiocyanate + TX, 2-(4,5-dimethyl-1,3-dioxolan-2-yl)phenyl methylcarbamate + TX, 2-(4-chloro-3,5-xylyloxy)ethanol + TX, 2-chlorovinyl diethyl phosphate + TX, 2-imidazolidone + TX, 2- isovalerylindan-1,3-dione + TX, 2-methyl(prop-2-ynyl)aminophenyl methylcarbamate + TX, 2- thiocyanatoethyl laurate + TX, 3-bromo-1-chloroprop-1-ene + TX, 3-methyl-1-phenylpyrazol-5-yl dimethyl-carbamate + TX, 4-methyl(prop-2-ynyl)amino-3,5-xylyl methylcarbamate + TX, 5,5-dimethyl-3- oxocyclohex-1-enyl dimethylcarbamate + TX, acethion + TX, acrylonitrile + TX, aldrin + TX, allosamidin + TX, allyxycarb + TX, alpha-ecdysone + TX, aluminium phosphide + TX, aminocarb + TX, anabasine + TX, athidathion + TX, azamethiphos + TX, Bacillus thuringiensis delta endotoxins + TX, barium hexafluorosilicate + TX, barium polysulfide + TX, barthrin + TX, Bayer 22/190 + TX, Bayer 22408 + TX, beta-cyfluthrin + TX, beta-cypermethrin + TX, bioethanomethrin + TX, biopermethrin + TX, bis(2- chloroethyl) ether + TX, borax + TX, bromfenvinfos + TX, bromo-DDT + TX, bufencarb + TX, butacarb + TX, butathiofos + TX, butonate + TX, calcium arsenate + TX, calcium cyanide + TX, carbon disulfide + TX, carbon tetrachloride + TX, cartap hydrochloride + TX, cevadine + TX, chlorbicyclen + TX, chlordane + TX, chlordecone + TX, chloroform + TX, chloropicrin + TX, chlorphoxim + TX, chlorprazophos + TX, cis-resmethrin + TX, cismethrin + TX, clocythrin + TX, copper acetoarsenite + TX, copper arsenate + TX, copper oleate + TX, coumithoate + TX, cryolite + TX, CS 708 + TX, cyanofenphos + TX, cyanophos + TX, cyclethrin + TX, cythioate + TX, d-tetramethrin + TX, DAEP + TX, dazomet + TX, decarbofuran + TX, diamidafos + TX, dicapthon + TX, dichlofenthion + TX, dicresyl + TX, dicyclanil + TX, dieldrin + TX, diethyl 5-methylpyrazol-3-yl phosphate + TX, dilor + TX, dimefluthrin + TX, dimetan + TX, dimethrin + TX, dimethylvinphos + TX, dimetilan + TX, dinoprop + TX, dinosam + TX, dinoseb + TX, diofenolan + TX, dioxabenzofos + TX, dithicrofos + TX, DSP + TX, ecdysterone + TX, EI 1642 + TX,
EMPC + TX, EPBP + TX, etaphos + TX, ethiofencarb + TX, ethyl formate + TX, ethylene dibromide + TX, ethylene dichloride + TX, ethylene oxide + TX, EXD + TX, fenchlorphos + TX, fenethacarb + TX, fenitrothion + TX, fenoxacrim + TX, fenpirithrin + TX, fensulfothion + TX, fenthion-ethyl + TX, flucofuron + TX, fosmethilan + TX, fospirate + TX, fosthietan + TX, furathiocarb + TX, furethrin + TX, guazatine + TX, guazatine acetates + TX, sodium tetrathiocarbonate + TX, halfenprox + TX, HCH + TX, HEOD + TX, heptachlor + TX, heterophos + TX, HHDN + TX, hydrogen cyanide + TX, hyquincarb + TX, IPSP + TX, isazofos + TX, isobenzan + TX, isodrin + TX, isofenphos + TX, isolane + TX, isoprothiolane + TX, isoxathion + TX, juvenile hormone I + TX, juvenile hormone II + TX, juvenile hormone III + TX, kelevan + TX, kinoprene + TX, lead arsenate + TX, leptophos + TX, lirimfos + TX, lythidathion + TX, m-cumenyl methylcarbamate + TX, magnesium phosphide + TX, mazidox + TX, mecarphon + TX, menazon + TX, mercurous chloride + TX, mesulfenfos + TX, metam + TX, metam-potassium + TX, metam-sodium + TX, methanesulfonyl fluoride + TX, methocrotophos + TX, methoprene + TX, methothrin + TX, methoxychlor + TX, methyl isothiocyanate + TX, methylchloroform + TX, methylene chloride + TX, metoxadiazone + TX, mirex + TX, naftalofos + TX, naphthalene + TX, NC-170 + TX, nicotine + TX, nicotine sulfate + TX, nithiazine + TX, nornicotine + TX, O-5-dichloro-4-iodophenyl O-ethyl ethylphosphonothioate + TX, O,O-diethyl O-4-methyl-2-oxo-2H-chromen-7-yl phosphorothioate + TX, O,O-diethyl O-6-methyl-2-propylpyrimidin-4-yl phosphorothioate + TX, O,O,O',O'-tetrapropyl dithiopyrophosphate + TX, oleic acid + TX, para-dichlorobenzene + TX, parathion-methyl + TX, pentachlorophenol + TX, pentachlorophenyl laurate + TX, PH 60-38 + TX, phenkapton + TX, phosnichlor + TX, phosphine + TX, phoxim-methyl + TX, pirimetaphos + TX, polychlorodicyclopentadiene isomers + TX, potassium arsenite + TX, potassium thiocyanate + TX, precocene I + TX, precocene II + TX, precocene III + TX, primidophos + TX, profluthrin + TX, promecarb + TX, prothiofos + TX, pyrazophos + TX, pyresmethrin + TX, quassia + TX, quinalphos-methyl + TX, quinothion + TX, rafoxanide + TX, resmethrin + TX, rotenone + TX, kadethrin + TX, ryania + TX, ryanodine + TX, sabadilla + TX, schradan + TX, sebufos + TX, SI-0009 + TX, thiapronil + TX, sodium arsenite + TX, sodium cyanide + TX, sodium fluoride + TX, sodium hexafluorosilicate + TX, sodium pentachlorophenoxide + TX, sodium selenate + TX, sodium thiocyanate + TX, sulcofuron + TX, sulcofuron-sodium + TX, sulfuryl fluoride + TX, sulprofos + TX, tar oils + TX, tazimcarb + TX, TDE + TX, tebupirimfos + TX, temephos + TX, terallethrin + TX, tetrachloroethane + TX, thicrofos + TX, thiocyclam + TX, thiocyclam hydrogen oxalate + TX, thionazin + TX, thiosultap + TX, thiosultap-sodium + TX, tralomethrin + TX, transpermethrin + TX, triazamate + TX, trichlormetaphos-3 + TX, trichloronat + TX, trimethacarb + TX, tolprocarb + TX, triclopyricarb + TX, triprene + TX, veratridine + TX, veratrine + TX, XMC + TX, zetamethrin + TX, zinc phosphide + TX, zolaprofos + TX, meperfluthrin + TX, tetramethylfluthrin + TX, bis(tributyltin) oxide + TX, bromoacetamide + TX, ferric phosphate + TX, niclosamide-olamine + TX, tributyltin oxide + TX, pyrimorph + TX, trifenmorph + TX, 1,2-dibromo-3-chloropropane + TX, 1,3-dichloropropene + TX, 3,4- dichlorotetrahydrothio-phene 1,1-dioxide + TX, 3-(4-chlorophenyl)-5-methylrhodanine + TX, 5-methyl-6- thioxo-1,3,5-thiadiazinan-3-ylacetic acid + TX, 6-isopentenylaminopurine + TX, anisiflupurin + TX, benclothiaz + TX, cytokinins + TX, DCIP + TX, furfural + TX, isamidofos + TX, kinetin + TX, Myrothecium verrucaria composition + TX, tetrachlorothiophene + TX, xylenols + TX, zeatin + TX, potassium ethylxanthate + TX ,acibenzolar + TX, acibenzolar-S-methyl + TX, Reynoutria sachalinensis extract + TX, alpha-chlorohydrin + TX, antu + TX, barium carbonate + TX, bisthiosemi + TX, brodifacoum + TX, bromadiolone + TX, bromethalin + TX, chlorophacinone + TX, cholecalciferol + TX, coumachlor + TX,
coumafuryl + TX, coumatetralyl + TX, crimidine + TX, difenacoum + TX, difethialone + TX, diphacinone + TX, ergocalciferol + TX, flocoumafen + TX, fluoroacetamide + TX, flupropadine + TX, flupropadine hydrochloride + TX, norbormide + TX, phosacetim + TX, phosphorus + TX, pindone + TX, pyrinuron + TX, scilliroside + TX, -sodium fluoroacetate + TX, thallium sulfate + TX, warfarin + TX, -2-(2- butoxyethoxy)ethyl piperonylate + TX, 5-(1,3-benzodioxol-5-yl)-3-hexylcyclohex-2-enone + TX, farnesol with nerolidol + TX, verbutin + TX, MGK 264 + TX, piperonyl butoxide + TX, piprotal + TX, propyl isomer + TX, S421 + TX, sesamex + TX, sesasmolin + TX, sulfoxide + TX, anthraquinone + TX, copper naphthenate + TX, copper oxychloride + TX, dicyclopentadiene + TX, thiram + TX, zinc naphthenate + TX, ziram + TX, imanin + TX, ribavirin + TX, chloroinconazide + TX, mercuric oxide + TX, thiophanate- methyl + TX, azaconazole + TX, bitertanol + TX, bromuconazole + TX, cyproconazole + TX, difenoconazole + TX, diniconazole -+ TX, epoxiconazole + TX, fenbuconazole + TX, fluquinconazole + TX, flusilazole + TX, flutriafol + TX, furametpyr + TX, hexaconazole + TX, imazalil- + TX, imiben-conazole + TX, ipconazole + TX, metconazole + TX, myclobutanil + TX, paclobutrazole + TX, pefurazoate + TX, penconazole + TX, prothioconazole + TX, pyrifenox + TX, prochloraz + TX, propiconazole + TX, pyrisoxazole + TX, -simeconazole + TX, tebucon-azole + TX, tetraconazole + TX, triadimefon + TX, triadimenol + TX, triflumizole + TX, triticonazole + TX, ancymidol + TX, fenarimol + TX, nuarimol + TX, bupirimate + TX, dimethirimol + TX, ethirimol + TX, dodemorph + TX, fenpropidin + TX, fenpropimorph + TX, spiroxamine + TX, tridemorph + TX, cyprodinil + TX, mepanipyrim + TX, pyrimethanil + TX, fenpiclonil + TX, fludioxonil + TX, benalaxyl + TX, furalaxyl + TX, metalaxyl + TX, R- metalaxyl + TX, ofurace + TX, oxadixyl + TX, carbendazim + TX, debacarb + TX, fuberidazole -+ TX, thiabendazole + TX, chlozolinate + TX, dichlozoline + TX, myclozoline- + TX, procymidone + TX, vinclozoline + TX, boscalid + TX, carboxin + TX, fenfuram + TX, flutolanil + TX, mepronil + TX, oxycarboxin + TX, penthiopyrad + TX, thifluzamide + TX, dodine + TX, iminoctadine + TX, azoxystrobin + TX, dimoxystrobin + TX, enestroburin + TX, fenaminstrobin + TX, flufenoxystrobin + TX, fluoxastrobin + TX, kresoxim--methyl + TX, metominostrobin + TX, trifloxystrobin + TX, orysastrobin + TX, picoxystrobin + TX, pyraclostrobin + TX, pyrametostrobin + TX, pyraoxystrobin + TX, ferbam + TX, mancozeb + TX, maneb + TX, metiram + TX, propineb + TX, zineb + TX, captafol + TX, captan + TX, fluoroimide + TX, folpet + TX, tolylfluanid + TX, bordeaux mixture + TX, copper oxide + TX, mancopper + TX, oxine-copper + TX, nitrothal-isopropyl + TX, edifenphos + TX, iprobenphos + TX, phosdiphen + TX, tolclofos-methyl + TX, anilazine + TX, benthiavalicarb + TX, blasticidin-S + TX, chloroneb -+ TX, chloro-tha-lonil + TX, cyflufenamid + TX, cymoxanil + TX, cyclobutrifluram + TX, diclocymet + TX, diclomezine + TX, dicloran + TX, diethofencarb + TX, dimethomorph -+ TX, flumorph + TX, dithianon + TX, ethaboxam + TX, etridiazole + TX, famoxadone + TX, fenamidone + TX, fenoxanil + TX, ferimzone + TX, fluazinam + TX, flumetylsulforim + TX, fluopicolide + TX, fluoxytioconazole + TX, flusulfamide + TX, fluxapyroxad + TX, fenhexamid + TX, fosetyl-aluminium -+ TX, hymexazol + TX, iprovalicarb + TX, cyazofamid + TX, methasulfocarb + TX, metrafenone + TX, pencycuron + TX, phthalide + TX, polyoxins + TX, propamocarb + TX, pyribencarb + TX, proquinazid + TX, pyroquilon + TX, pyriofenone + TX, quinoxyfen + TX, quintozene + TX, tiadinil + TX, triazoxide + TX, tricyclazole + TX, triforine + TX, validamycin + TX, valifenalate + TX, zoxamide + TX, mandipropamid + TX, flubeneteram + TX, isopyrazam + TX, sedaxane + TX, benzovindiflupyr + TX, pydiflumetofen + TX, 3-difluoromethyl-1- methyl-1H-pyrazole-4-carboxylic acid (3',4',5'-trifluoro-biphenyl-2-yl)-amide + TX, isoflucypram + TX, isotianil + TX, dipymetitrone + TX, 6-ethyl-5,7-dioxo-pyrrolo[4,5][1,4]dithiino[1,2-c]isothiazole-3-
carbonitrile + TX, 2-(difluoromethyl)-N-[3-ethyl-1,1-dimethyl-indan-4-yl]pyridine-3-carboxamide + TX, 4- (2,6-difluorophenyl)-6-methyl-5-phenyl-pyridazine-3-carbonitrile + TX, (R)-3-(difluoromethyl)-1-methyl- N-[1,1,3-trimethylindan-4-yl]pyrazole-4-carboxamide + TX, 4-(2-bromo-4-fluoro-phenyl)-N-(2-chloro-6- fluoro-phenyl)-2,5-dimethyl-pyrazol-3-amine + TX, 4- (2- bromo- 4- fluorophenyl) - N- (2- chloro- 6- fluorophenyl) - 1, 3- dimethyl- 1H- pyrazol- 5- amine + TX, fluindapyr + TX, coumethoxystrobin (jiaxiangjunzhi) + TX, lvbenmixianan + TX, dichlobentiazox + TX, mandestrobin + TX, 3-(4,4-difluoro- 3,4-dihydro-3,3-dimethylisoquinolin-1-yl)quinolone + TX, 2-[2-fluoro-6-[(8-fluoro-2-methyl-3- quinolyl)oxy]phenyl]propan-2-ol + TX, oxathiapiprolin + TX, tert-butyl N-[6-[[[(1-methyltetrazol-5-yl)- phenyl-methylene]amino]oxymethyl]-2-pyridyl]carbamate + TX, pyraziflumid + TX, inpyrfluxam + TX, trolprocarb + TX, mefentrifluconazole + TX, ipfentrifluconazole+ TX, 2-(difluoromethyl)-N-[(3R)-3-ethyl- 1,1-dimethyl-indan-4-yl]pyridine-3-carboxamide + TX, N'-(2,5-dimethyl-4-phenoxy-phenyl)-N-ethyl-N- methyl-formamidine + TX, N'-[4-(4,5-dichlorothiazol-2-yl)oxy-2,5-dimethyl-phenyl]-N-ethyl-N-methyl- formamidine + TX, [2-[3-[2-[1-[2-[3,5-bis(difluoromethyl)pyrazol-1-yl]acetyl]-4-piperidyl]thiazol-4-yl]-4,5- dihydroisoxazol-5-yl]-3-chloro-phenyl] methanesulfonate + TX, but-3-ynyl N-[6-[[(Z)-[(1-methyltetrazol- 5-yl)-phenyl-methylene]amino]oxymethyl]-2-pyridyl]carbamate + TX, methyl N-[[5-[4-(2,4- dimethylphenyl)triazol-2-yl]-2-methyl-phenyl]methyl]carbamate + TX, 3-chloro-6-methyl-5-phenyl-4- (2,4,6-trifluorophenyl)pyridazine + TX, pyridachlometyl + TX, 3-(difluoromethyl)-1-methyl-N-[1,1,3- trimethylindan-4-yl]pyrazole-4-carboxamide + TX, 1-[2-[[1-(4-chlorophenyl)pyrazol-3-yl]oxymethyl]-3- methyl-phenyl]-4-methyl-tetrazol-5-one + TX, 1-methyl-4-[3-methyl-2-[[2-methyl-4-(3,4,5- trimethylpyrazol-1-yl)phenoxy]methyl]phenyl]tetrazol-5-one + TX, aminopyrifen + TX, ametoctradin + TX, amisulbrom + TX, penflufen + TX, (Z,2E)-5-[1-(4-chlorophenyl)pyrazol-3-yl]oxy-2-methoxyimino- N,3-dimethyl-pent-3-enamide + TX, florylpicoxamid + TX, fenpicoxamid + TX, metarylpicoxamid + TX, tebufloquin + TX, ipflufenoquin + TX, quinofumelin + TX, isofetamid + TX, ethyl 1-[[4-[[2-(trifluoromethyl)- 1,3-dioxolan-2-yl]methoxy]phenyl]methyl]pyrazole-3-carboxylate + TX (may be prepared from the methods described in WO 2020/056090), ethyl 1-[[4-[(Z)-2-ethoxy-3,3,3-trifluoro-prop-1- enoxy]phenyl]methyl]pyrazole-3-carboxylate + TX (may be prepared from the methods described in WO 2020/056090), methyl N-[[4-[1-(4-cyclopropyl-2,6-difluoro-phenyl)pyrazol-4-yl]-2-methyl- phenyl]methyl]carbamate + TX (may be prepared from the methods described in WO 2020/097012), methyl N-[[4-[1-(2,6-difluoro-4-isopropyl-phenyl)pyrazol-4-yl]-2-methyl-phenyl]methyl]carbamate + TX (may be prepared from the methods described in WO 2020/097012), 6-chloro-3-(3-cyclopropyl-2-fluoro- phenoxy)-N-[2-(2,4-dimethylphenyl)-2,2-difluoro-ethyl]-5-methyl-pyridazine-4-carboxamide + TX (may be prepared from the methods described in WO 2020/109391), 6-chloro-N-[2-(2-chloro-4-methyl- phenyl)-2,2-difluoro-ethyl]-3-(3-cyclopropyl-2-fluoro-phenoxy)-5-methyl-pyridazine-4-carboxamide + TX (may be prepared from the methods described in WO 2020/109391), 6-chloro-3-(3-cyclopropyl-2-fluoro- phenoxy)-N-[2-(3,4-dimethylphenyl)-2,2-difluoro-ethyl]-5-methyl-pyridazine-4-carboxamide + TX (may be prepared from the methods described in WO 2020/109391), N-[2-[2,4-dichloro-phenoxy]phenyl]-3- (difluoromethyl)-1-methyl-pyrazole-4-carboxamide + TX, N-[2-[2-chloro-4- (trifluoromethyl)phenoxy]phenyl]-3-(difluoromethyl)-1-methyl-pyrazole-4-carboxamide + TX, benzothiostrobin + TX, phenamacril + TX, 5-amino-1,3,4-thiadiazole-2-thiol zinc salt (2:1) + TX, fluopyram + TX, flufenoxadiazam + TX, flutianil + TX, fluopimomide + TX, pyrapropoyne + TX, picarbutrazox + TX, 2-(difluoromethyl)-N-(3-ethyl-1,1-dimethyl-indan-4-yl)pyridine-3-carboxamide + TX, 2- (difluoromethyl) - N- ((3R) - 1, 1, 3- trimethylindan- 4- yl) pyridine- 3- carboxamide + TX, 4-[[6-[2-(2,4-
difluorophenyl)-1,1-difluoro-2-hydroxy-3-(1,2,4-triazol-1-yl)propyl]-3-pyridyl]oxy]benzonitrile + TX, metyltetraprole + TX, 2- (difluoromethyl) - N- ((3R) - 1, 1, 3- trimethylindan- 4- yl) pyridine- 3- carboxamide + TX, α- (1, 1- dimethylethyl) - α- [4'- (trifluoromethoxy) [1, 1'- biphenyl] - 4- yl] -5- pyrimidinemethanol + TX, fluoxapiprolin + TX, enoxastrobin + TX, methyl (Z)-3-methoxy-2-[2-methyl-5- [4-(trifluoromethyl)triazol-2-yl]phenoxy]prop-2-enoate + TX, methyl (Z)-3-methoxy-2-[2-methyl-5-(4- propyltriazol-2-yl)phenoxy]prop-2-enoate + TX, methyl (Z)-2-[5-(3-isopropylpyrazol-1-yl)-2-methyl- phenoxy]-3-methoxy-prop-2-enoate + TX, methyl (Z)-3-methoxy-2-[2-methyl-5-(3-propylpyrazol-1- yl)phenoxy]prop-2-enoate + TX, methyl (Z)-3-methoxy-2-[2-methyl-5-[3-(trifluoromethyl)pyrazol-1- yl]phenoxy]prop-2-enoate + TX (these compounds may be prepared from the methods described in WO2020/079111), methyl (Z)-2-(5-cyclohexyl-2-methyl-phenoxy)-3-methoxy-prop-2-enoate + TX, methyl (Z)-2-(5-cyclopentyl-2-methyl-phenoxy)-3-methoxy-prop-2-enoate + TX (these compounds may be prepared from the methods described in WO2020/193387), 4-[[6-[2-(2,4-difluorophenyl)-1,1-difluoro- 2-hydroxy-3-(1,2,4-triazol-1-yl)propyl]-3-pyridyl]oxy] benzonitrile + TX, 4-[[6-[2-(2,4-difluorophenyl)-1,1- difluoro-2-hydroxy-3-(5-sulfanyl-1,2,4-triazol-1-yl)propyl]-3-pyridyl]oxy] benzonitrile + TX, 4-[[6-[2-(2,4- difluorophenyl)-1,1-difluoro-2-hydroxy-3-(5-thioxo-4H-1,2,4-triazol-1-yl)propyl]-3- pyridyl]oxy]benzonitrile + TX, trinexapac + TX, coumoxystrobin + TX, zhongshengmycin + TX, thiodiazole copper + TX, zinc thiazole + TX, amectotractin + TX, iprodione + TX, seboctylamine + TX; N'-[5-bromo-2-methyl-6-[(1S)-1-methyl-2-propoxy-ethoxy]-3-pyridyl]-N-ethyl-N-methyl-formamidine + TX, N'-[5-bromo-2-methyl-6-[(1R)-1-methyl-2-propoxy-ethoxy]-3-pyridyl]-N-ethyl-N-methyl-formamidine + TX, N'-[5-bromo-2-methyl-6-(1-methyl-2-propoxy-ethoxy)-3-pyridyl]-N-ethyl-N-methyl-formamidine + TX, N'-[5-chloro-2-methyl-6-(1-methyl-2-propoxy-ethoxy)-3-pyridyl]-N-ethyl-N-methyl-formamidine + TX, N'-[5-bromo-2-methyl-6-(1-methyl-2-propoxy-ethoxy)-3-pyridyl]-N-isopropyl-N-methyl-formamidine + TX (these compounds may be prepared from the methods described in WO2015/155075); N'-[5- bromo-2-methyl-6-(2-propoxypropoxy)-3-pyridyl]-N-ethyl-N-methyl-formamidine + TX (this compound may be prepared from the methods described in IPCOM000249876D); N-isopropyl-N’-[5-methoxy-2- methyl-4-(2,2,2-trifluoro-1-hydroxy-1-phenyl-ethyl)phenyl]-N-methyl-formamidine+ TX, N’-[4-(1- cyclopropyl-2,2,2-trifluoro-1-hydroxy-ethyl)-5-methoxy-2-methyl-phenyl]-N-isopropyl-N-methyl- formamidine + TX (these compounds may be prepared from the methods described in WO2018/228896); N-ethyl-N’-[5-methoxy-2-methyl-4-[(2-trifluoromethyl)oxetan-2-yl]phenyl]-N-methyl- formamidine + TX, N-ethyl-N’-[5-methoxy-2-methyl-4-[(2-trifuoromethyl)tetrahydrofuran-2-yl]phenyl]-N- methyl-formamidine + TX (these compounds may be prepared from the methods described in WO2019/110427); N-[(1R)-1-benzyl-3-chloro-1-methyl-but-3-enyl]-8-fluoro-quinoline-3-carboxamide + TX, N-[(1S)-1-benzyl-3-chloro-1-methyl-but-3-enyl]-8-fluoro-quinoline-3-carboxamide + TX, N-[(1R)-1- benzyl-3,3,3-trifluoro-1-methyl-propyl]-8-fluoro-quinoline-3-carboxamide + TX, N-[(1S)-1-benzyl-3,3,3- trifluoro-1-methyl-propyl]-8-fluoro-quinoline-3-carboxamide + TX, N-[(1R)-1-benzyl-1,3-dimethyl-butyl]- 7,8-difluoro-quinoline-3-carboxamide + TX, N-[(1S)-1-benzyl-1,3-dimethyl-butyl]-7,8-difluoro-quinoline- 3-carboxamide + TX, 8-fluoro-N-[(1R)-1-[(3-fluorophenyl)methyl]-1,3-dimethyl-butyl]quinoline-3- carboxamide + TX, 8-fluoro-N-[(1S)-1-[(3-fluorophenyl)methyl]-1,3-dimethyl-butyl]quinoline-3- carboxamide + TX, N-[(1R)-1-benzyl-1,3-dimethyl-butyl]-8-fluoro-quinoline-3-carboxamide + TX, N- [(1S)-1-benzyl-1,3-dimethyl-butyl]-8-fluoro-quinoline-3-carboxamide + TX, N-((1R)-1-benzyl-3-chloro-1- methyl-but-3-enyl)-8-fluoro-quinoline-3-carboxamide + TX, N-((1S)-1-benzyl-3-chloro-1-methyl-but-3- enyl)-8-fluoro-quinoline-3-carboxamide + TX (these compounds may be prepared from the methods
described in WO2017/153380); 1-(6,7-dimethylpyrazolo[1,5-a]pyridin-3-yl)-4,4,5-trifluoro-3,3-dimethyl- isoquinoline + TX, 1-(6,7-dimethylpyrazolo[1,5-a]pyridin-3-yl)-4,4,6-trifluoro-3,3-dimethyl-isoquinoline + TX, 4,4-difluoro-3,3-dimethyl-1-(6-methylpyrazolo[1,5-a]pyridin-3-yl)isoquinoline + TX, 4,4-difluoro-3,3- dimethyl-1-(7-methylpyrazolo[1,5-a]pyridin-3-yl)isoquinoline + TX, 1-(6-chloro-7-methyl-pyrazolo[1,5- a]pyridin-3-yl)-4,4-difluoro-3,3-dimethyl-isoquinoline + TX (these compounds may be prepared from the methods described in WO2017/025510); 1-(4,5-dimethylbenzimidazol-1-yl)-4,4,5-trifluoro-3,3-dimethyl- isoquinoline + TX, 1-(4,5-dimethylbenzimidazol-1-yl)-4,4-difluoro-3,3-dimethyl-isoquinoline + TX, 6- chloro-4,4-difluoro-3,3-dimethyl-1-(4-methylbenzimidazol-1-yl)isoquinoline + TX, 4,4-difluoro-1-(5- fluoro-4-methyl-benzimidazol-1-yl)-3,3-dimethyl-isoquinoline + TX, 3-(4,4-difluoro-3,3-dimethyl-1- isoquinolyl)-7,8-dihydro-6H-cyclopenta[e]benzimidazole + TX (these compounds may be prepared from the methods described in WO2016/156085); N-methoxy-N-[[4-[5-(trifluoromethyl)-1,2,4-oxadiazol-3- yl]phenyl]methyl]cyclopropanecarboxamide + TX, N,2-dimethoxy-N-[[4-[5-(trifluoromethyl)-1,2,4- oxadiazol-3-yl]phenyl]methyl]propanamide + TX, N-ethyl-2-methyl-N-[[4-[5-(trifluoromethyl)-1,2,4- oxadiazol-3-yl]phenyl]methyl]propanamide + TX, 1-methoxy-3-methyl-1-[[4-[5-(trifluoromethyl)-1,2,4- oxadiazol-3-yl]phenyl]methyl]urea + TX, 1,3-dimethoxy-1-[[4-[5-(trifluoromethyl)-1,2,4-oxadiazol-3- yl]phenyl]methyl]urea + TX, 3-ethyl-1-methoxy-1-[[4-[5-(trifluoromethyl)-1,2,4-oxadiazol-3- yl]phenyl]methyl]urea + TX, N-[[4-[5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl]phenyl]methyl]propanamide + TX, 4,4-dimethyl-2-[[4-[5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl]phenyl]methyl]isoxazolidin-3-one + TX, 5,5-dimethyl-2-[[4-[5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl]phenyl]methyl]isoxazolidin-3-one + TX, ethyl 1-[[4-[5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl]phenyl]methyl]pyrazole-4-carboxylate + TX, N,N-dimethyl- 1-[[4-[5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl]phenyl]methyl]-1,2,4-triazol-3-amine + TX. The compounds in this paragraph may be prepared from the methods described in WO 2017/055473, WO 2017/055469, WO 2017/093348 and WO 2017/118689; 2-[6-(4-chlorophenoxy)-2-(trifluoromethyl)-3- pyridyl]-1-(1,2,4-triazol-1-yl)propan-2-ol + TX (this compound may be prepared from the methods described in WO 2017/029179); 2-[6-(4-bromophenoxy)-2-(trifluoromethyl)-3-pyridyl]-1-(1,2,4-triazol-1- yl)propan-2-ol + TX (this compound may be prepared from the methods described in WO 2017/029179); 3-[2-(1-chlorocyclopropyl)-3-(2-fluorophenyl)-2-hydroxy-propyl]imidazole-4-carbonitrile + TX (this compound may be prepared from the methods described in WO 2016/156290); 3-[2-(1- chlorocyclopropyl)-3-(3-chloro-2-fluoro-phenyl)-2-hydroxy-propyl]imidazole-4-carbonitrile + TX (this compound may be prepared from the methods described in WO 2016/156290); (4- phenoxyphenyl)methyl 2-amino-6-methyl-pyridine-3-carboxylate + TX (this compound may be prepared from the methods described in WO 2014/006945); 2,6-Dimethyl-1H,5H-[1,4]dithiino[2,3-c:5,6- c']dipyrrole-1,3,5,7(2H,6H)-tetrone + TX (this compound may be prepared from the methods described in WO 2011/138281); N-methyl-4-[5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl]benzenecarbothioamide + TX; N-methyl-4-[5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl]benzamide + TX; (Z,2E)-5-[1-(2,4- dichlorophenyl)pyrazol-3-yl]oxy-2-methoxyimino-N,3-dimethyl-pent-3-enamide + TX (this compound may be prepared from the methods described in WO 2018/153707); N'-(2-chloro-5-methyl-4-phenoxy- phenyl)-N-ethyl-N-methyl-formamidine + TX; N'-[2-chloro-4-(2-fluorophenoxy)-5-methyl-phenyl]-N- ethyl-N-methyl-formamidine + TX (this compound may be prepared from the methods described in WO 2016/202742); 2-(difluoromethyl)-N-[(3S)-3-ethyl-1,1-dimethyl-indan-4-yl]pyridine-3-carboxamide + TX (this compound may be prepared from the methods described in WO 2014/095675); (5-methyl-2- pyridyl)-[4-[5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl]phenyl]methanone + TX, (3-methylisoxazol-5-yl)-[4-
[5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl]phenyl]methanone + TX (these compounds may be prepared from the methods described in WO 2017/220485); 2-oxo-N-propyl-2-[4-[5-(trifluoromethyl)-1,2,4- oxadiazol-3-yl]phenyl]acetamide + TX (this compound may be prepared from the methods described in WO 2018/065414); ethyl 1-[[5-[5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl]-2-thienyl]methyl]pyrazole-4- carboxylate + TX (this compound may be prepared from the methods described in WO 2018/158365); 2,2-difluoro-N-methyl-2-[4-[5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl]phenyl]acetamide + TX, N-[(E)- methoxyiminomethyl]-4-[5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl]benzamide + TX, N-[(Z)- methoxyiminomethyl]-4-[5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl]benzamide + TX, N-[N-methoxy-C- methyl-carbonimidoyl]-4-[5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl]benzamide + TX (these compounds may be prepared from the methods described in WO 2018/202428); microbials including: Acinetobacter lwoffii + TX, Acremonium alternatum + TX + TX, Acremonium cephalosporium + TX + TX, Acremonium diospyri + TX, Acremonium obclavatum + TX, Adoxophyes orana granulovirus (AdoxGV) (Capex®) + TX, Agrobacterium radiobacter strain K84 (Galltrol-A®) + TX, Alternaria alternate + TX, Alternaria cassia + TX, Alternaria destruens (Smolder®) + TX, Ampelomyces quisqualis (AQ10®) + TX, Aspergillus flavus AF36 (AF36®) + TX, Aspergillus flavus NRRL 21882 (Aflaguard®) + TX, Aspergillus spp. + TX, Aureobasidium pullulans + TX, Azospirillum (MicroAZ®, TAZO B®) + TX, Azotobacter + TX, Azotobacter chroocuccum (Azotomeal®) + TX, Azotobacter cysts (Bionatural Blooming Blossoms®) + TX, Bacillus amyloliquefaciens + TX, Bacillus cereus + TX, Bacillus chitinosporus strain CM-1 + TX, Bacillus chitinosporus strain AQ746 + TX, Bacillus licheniformis strain HB-2 (e.g, Biostart™, formerly Rhizoboost®) + TX, Bacillus licheniformis strain 3086 (EcoGuard®, Green Releaf®) + TX, Bacillus circulans + TX, Bacillus firmus (BioSafe®, BioNem-WP®, VOTiVO®) + TX, Bacillus firmus strain I-1582 + TX, Bacillus macerans + TX, Bacillus marismortui + TX, Bacillus megaterium + TX, Bacillus mycoides strain AQ726 + TX, Bacillus papillae (Milky Spore Powder®) + TX, Bacillus pumilus spp. + TX, Bacillus pumilus strain GB34 (Yield Shield®) + TX, Bacillus pumilus strain AQ717 + TX, Bacillus pumilus strain QST 2808 (Sonata®, Ballad Plus®) + TX, Bacillus spahericus (VectoLex®) + TX, Bacillus spp. + TX, Bacillus spp. strain AQ175 + TX, Bacillus spp. strain AQ177 + TX, Bacillus spp. strain AQ178 + TX, Bacillus subtilis strain QST 713 (CEASE®, Serenade®, Rhapsody®) + TX, Bacillus subtilis strain QST 714 (JAZZ®) + TX, Bacillus subtilis strain AQ153 + TX, Bacillus subtilis strain AQ743 + TX, Bacillus subtilis strain QST3002 + TX, Bacillus subtilis strain QST3004 + TX, Bacillus subtilis var. amyloliquefaciens strain FZB24 (Taegro®, Rhizopro®) + TX, Bacillus thuringiensis Cry 2Ae + TX, Bacillus thuringiensis Cry1Ab + TX, Bacillus thuringiensis aizawai GC 91 (Agree®) + TX, Bacillus thuringiensis israelensis (BMP123®, Aquabac®, VectoBac®) + TX, Bacillus thuringiensis kurstaki (Javelin®, Deliver®, CryMax®, Bonide®, Scutella WP®, Turilav WP®, Astuto®, Dipel WP®, Biobit®, Foray®) + TX, Bacillus thuringiensis kurstaki BMP 123 (Baritone®) + TX, Bacillus thuringiensis kurstaki HD-1 (Bioprotec-CAF / 3P®) + TX, Bacillus thuringiensis strain BD#32 + TX, Bacillus thuringiensis strain AQ52 + TX, Bacillus thuringiensis var. aizawai (XenTari®, DiPel®) + TX, bacteria spp. (GROWMEND®, GROWSWEET®X, Shootup®) + TX, bacteriophage of Clavipacter michiganensis (AgriPhage®) + TX, Bakflor® + TX, Beauveria bassiana (Beaugenic®, Brocaril WP®) + TX, Beauveria bassiana GHA (Mycotrol ES®, Mycotrol O®, BotaniGuard®) + TX, Beauveria brongniartii (Engerlingspilz®, Schweizer Beauveria®, Melocont®) + TX, Beauveria spp. + TX, Botrytis cineria + TX, Bradyrhizobium japonicum (TerraMax®) + TX, Brevibacillus brevis + TX, Bacillus thuringiensis
tenebrionis (Novodor®) + TX, BtBooster + TX, Burkholderia cepacia (Deny®, Intercept®, Blue Circle®) + TX, Burkholderia gladii + TX, Burkholderia gladioli + TX, Burkholderia spp. + TX, Canadian thistle fungus (CBH Canadian Bioherbicide®) + TX, Candida butyri + TX, Candida famata + TX, Candida fructus + TX, Candida glabrata + TX, Candida guilliermondii + TX, Candida melibiosica + TX, Candida oleophila strain O + TX, Candida parapsilosis + TX, Candida pelliculosa + TX, Candida pulcherrima + TX, Candida reukaufii + TX, Candida saitoana (Bio-Coat®, Biocure®) + TX, Candida sake + TX, Candida spp. + TX, Candida tenius + TX, Cedecea dravisae + TX, Cellulomonas flavigena + TX, Chaetomium cochliodes (Nova-Cide®) + TX, Chaetomium globosum (Nova-Cide®) + TX, Chromobacterium subtsugae strain PRAA4-1T (Grandevo®) + TX, Cladosporium cladosporioides + TX, Cladosporium oxysporum + TX, Cladosporium chlorocephalum + TX, Cladosporium spp. + TX, Cladosporium tenuissimum + TX, Clonostachys rosea (EndoFine®) + TX, Colletotrichum acutatum + TX, Coniothyrium minitans (Cotans WG®) + TX, Coniothyrium spp. + TX, Cryptococcus albidus (YIELDPLUS®) + TX, Cryptococcus humicola + TX, Cryptococcus infirmo-miniatus + TX, Cryptococcus laurentii + TX, Cryptophlebia leucotreta granulovirus (Cryptex®) + TX, Cupriavidus campinensis + TX, Cydia pomonella granulovirus (CYD-X®) + TX, Cydia pomonella granulovirus (Madex®, Madex® Plus, Madex® Max, Carpovirusine Evo2®) + TX, Cylindrobasidium laeve (Stumpout®) + TX, Cylindrocladium + TX, Debaryomyces hansenii + TX, Drechslera hawaiinensis + TX, Enterobacter cloacae + TX, Enterobacteriaceae + TX, Entomophtora virulenta (Vektor®) + TX, Epicoccum nigrum + TX, Epicoccum purpurascens + TX, Epicoccum spp. + TX, Filobasidium floriforme + TX, Fusarium acuminatum + TX, Fusarium chlamydosporum + TX, Fusarium oxysporum (Fusaclean®, Biofox C®) + TX, Fusarium proliferatum + TX, Fusarium spp. + TX, Galactomyces geotrichum + TX, Gliocladium catenulatum (Primastop®, Prestop®) + TX, Gliocladium roseum + TX, Gliocladium spp. (SoilGard®) + TX, Gliocladium virens (Soilgard®) + TX, Granulovirus (Granupom®) + TX, Halobacillus halophilus + TX, Halobacillus litoralis + TX, Halobacillus trueperi + TX, Halomonas spp. + TX, Halomonas subglaciescola + TX, Halovibrio variabilis + TX, Hanseniaspora uvarum + TX, Helicoverpa armigera nucleopolyhedrovirus (Helicovex®) + TX, Helicoverpa zea nuclear polyhedrosis virus (Gemstar®) + TX, Isoflavone – formononetin (Myconate®) + TX, Kloeckera apiculata + TX, Kloeckera spp. + TX, Lagenidium giganteum (Laginex®) + TX, Lecanicillium longisporum (Vertiblast®) + TX, Lecanicillium muscarium (Vertikil®) + TX, Lymantria Dispar nucleopolyhedrosis virus (Disparvirus®) + TX, Marinococcus halophilus + TX, Meira geulakonigii + TX, Metarhizium anisopliae (Met52®) + TX, Metarhizium anisopliae (Destruxin WP®) + TX, Metschnikowia fruticola (Shemer®) + TX, Metschnikowia pulcherrima + TX, Microdochium dimerum (Antibot®) + TX, Micromonospora coerulea + TX, Microsphaeropsis ochracea + TX, Muscodor albus 620 (Muscudor®) + TX, Muscodor roseus strain A3- 5 + TX, Mycorrhizae spp. (AMykor®, Root Maximizer®) + TX, Myrothecium verrucaria strain AARC- 0255 (DiTera®) + TX, BROS PLUS® + TX, Ophiostoma piliferum strain D97 (Sylvanex®) + TX, Paecilomyces farinosus + TX, Paecilomyces fumosoroseus (PFR-97®, PreFeRal®) + TX, Paecilomyces linacinus (Biostat WP®) + TX, Paecilomyces lilacinus strain 251 (MeloCon WG®) + TX, Paenibacillus polymyxa + TX, Pantoea agglomerans (BlightBan C9-1®) + TX, Pantoea spp. + TX, Pasteuria spp. (Econem®) + TX, Pasteuria nishizawae + TX, Penicillium aurantiogriseum + TX, Penicillium billai (Jumpstart®, TagTeam®) + TX, Penicillium brevicompactum + TX, Penicillium frequentans + TX, Penicillium griseofulvum + TX, Penicillium purpurogenum + TX, Penicillium spp. + TX, Penicillium viridicatum + TX, Phlebiopsis gigantean (Rotstop®) + TX, phosphate solubilizing bacteria
(Phosphomeal®) + TX, Phytophthora cryptogea + TX, Phytophthora palmivora (Devine®) + TX, Pichia anomala + TX, Pichia guilermondii + TX, Pichia membranaefaciens + TX, Pichia onychis + TX, Pichia stipites + TX, Pseudomonas aeruginosa + TX, Pseudomonas aureofasciens (Spot-Less Biofungicide®) + TX, Pseudomonas cepacia + TX, Pseudomonas chlororaphis (AtEze®) + TX, Pseudomonas corrugate + TX, Pseudomonas fluorescens strain A506 (BlightBan A506®) + TX, Pseudomonas putida + TX, Pseudomonas reactans + TX, Pseudomonas spp. + TX, Pseudomonas syringae (Bio-Save®) + TX, Pseudomonas viridiflava + TX, Pseudomons fluorescens (Zequanox®) + TX, Pseudozyma flocculosa strain PF-A22 UL (Sporodex L®) + TX, Puccinia canaliculata + TX, Puccinia thlaspeos (Wood Warrior®) + TX, Pythium paroecandrum + TX, Pythium oligandrum (Polygandron®, Polyversum®) + TX, Pythium periplocum + TX, Rhanella aquatilis + TX, Rhanella spp. + TX, Rhizobia (Dormal®, Vault®) + TX, Rhizoctonia + TX, Rhodococcus globerulus strain AQ719 + TX, Rhodosporidium diobovatum + TX, Rhodosporidium toruloides + TX, Rhodotorula spp. + TX, Rhodotorula glutinis + TX, Rhodotorula graminis + TX, Rhodotorula mucilagnosa + TX, Rhodotorula rubra + TX, Saccharomyces cerevisiae + TX, Salinococcus roseus + TX, Sclerotinia minor + TX, Sclerotinia minor (SARRITOR®) + TX, Scytalidium spp. + TX, Scytalidium uredinicola + TX, Spodoptera exigua nuclear polyhedrosis virus (Spod-X®, Spexit®) + TX, Serratia marcescens + TX, Serratia plymuthica + TX, Serratia spp. + TX, Sordaria fimicola + TX, Spodoptera littoralis nucleopolyhedrovirus (Littovir®) + TX, Sporobolomyces roseus + TX, Stenotrophomonas maltophilia + TX, Streptomyces ahygroscopicus + TX, Streptomyces albaduncus + TX, Streptomyces exfoliates + TX, Streptomyces galbus + TX, Streptomyces griseoplanus + TX, Streptomyces griseoviridis (Mycostop®) + TX, Streptomyces lydicus (Actinovate®) + TX, Streptomyces lydicus WYEC-108 (ActinoGrow®) + TX, Streptomyces violaceus + TX, Tilletiopsis minor + TX, Tilletiopsis spp. + TX, Trichoderma asperellum (T34 Biocontrol®) + TX, Trichoderma gamsii (Tenet®) + TX, Trichoderma atroviride (Plantmate®) + TX, Trichoderma hamatum TH 382 + TX, Trichoderma harzianum rifai (Mycostar®) + TX, Trichoderma harzianum T-22 (Trianum-P®, PlantShield HC®, RootShield®, Trianum-G®) + TX, Trichoderma harzianum T-39 (Trichodex®) + TX, Trichoderma inhamatum + TX, Trichoderma koningii + TX, Trichoderma spp. LC 52 (Sentinel®) + TX, Trichoderma lignorum + TX, Trichoderma longibrachiatum + TX, Trichoderma polysporum (Binab T®) + TX, Trichoderma taxi + TX, Trichoderma virens + TX, Trichoderma virens (formerly Gliocladium virens GL- 21) (SoilGuard®) + TX, Trichoderma viride + TX, Trichoderma viride strain ICC 080 (Remedier®) + TX, Trichosporon pullulans + TX, Trichosporon spp. + TX, Trichothecium spp. + TX, Trichothecium roseum + TX, Typhula phacorrhiza strain 94670 + TX, Typhula phacorrhiza strain 94671 + TX, Ulocladium atrum + TX, Ulocladium oudemansii (Botry-Zen®) + TX, Ustilago maydis + TX, various bacteria and supplementary micronutrients (Natural II®) + TX, various fungi (Millennium Microbes®) + TX, Verticillium chlamydosporium + TX, Verticillium lecanii (Mycotal®, Vertalec®) + TX, Vip3Aa20 (VIPtera®) + TX, Virgibaclillus marismortui + TX, Xanthomonas campestris pv. Poae (Camperico®) + TX, Xenorhabdus bovienii + TX, and Xenorhabdus nematophilus + TX; Plant extracts including: pine oil (Retenol®) + TX, azadirachtin (Plasma Neem Oil®, AzaGuard®, MeemAzal®, Molt-X®) + TX, Botanical IGR (Neemazad®, Neemix®) + TX, canola oil (Lilly Miller Vegol®) + TX, Chenopodium ambrosioides near ambrosioides (Requiem®) + TX, Chrysanthemum extract (Crisant®) + TX, extract of neem oil (Trilogy®) + TX, essentials oils of Labiatae (Botania®) + TX, extracts of clove rosemary peppermint and thyme oil (Garden insect killer®) + TX, Glycinebetaine (Greenstim®) + TX, garlic + TX, lemongrass oil (GreenMatch®) + TX, neem oil + TX, Nepeta cataria
(Catnip oil) + TX, Nepeta catarina + TX, nicotine + TX, oregano oil (MossBuster®) + TX, Pedaliaceae oil (Nematon®) + TX, pyrethrum + TX, Quillaja saponaria (NemaQ®) + TX, Reynoutria ® (Regalia®, Sakalia®) + TX, rotenone (Eco Roten®) + TX, Rutaceae plant extract (Soleo®) + TX, soybean oil (Ortho ecosense®) + TX, Melaleuca alternifolia extract (also called tea tree oil) (Timorex Gold®) + TX, thymus oil + TX, AGNIQUE® MMF + TX, BugOil® + TX, mixture of rosemary sesame pepermint thyme and cinnamon extracts (EF 300®) + TX, mixture of clove rosemary and peppermint extract (EF 400®) + TX, mixture of clove pepermint garlic oil and mint (Soil Shot®) + TX, kaolin (Screen®) + TX, storage glucam of brown algae (Laminarin®) +TX; pheromones including: blackheaded fireworm pheromone (3M Sprayable Blackheaded Fireworm Pheromone®) + TX, Codling Moth Pheromone (Paramount dispenser-(CM)/ Isomate C-Plus®) + TX, Grape Berry Moth Pheromone (3M MEC-GBM Sprayable Pheromone®) + TX, Leafroller pheromone (3M MEC – LR Sprayable Pheromone®) + TX, Muscamone (Snip7 Fly Bait®, Starbar Premium Fly Bait®) + TX, Oriental Fruit Moth Pheromone (3M oriental fruit moth sprayable pheromone®) + TX, Peachtree Borer Pheromone (Isomate-P®) + TX, Tomato Pinworm Pheromone (3M Sprayable pheromone®) + TX, Entostat powder (extract from palm tree) (Exosex CM®) + TX, (3E,8Z,11Z)-3,8,11- Tetradecatrienyl acetate + TX, (7Z,11Z,13E)-7,11,13-Hexadecatrienal + TX, (E,Z)-7,9-Dodecadien-1-yl acetate + TX, 2-Methyl-1-butanol + TX, Calcium acetate + TX, Scenturion® + TX, Biolure® + TX, Check- Mate® + TX, Lavandulyl senecioate + TX; Macrobials including: Aphelinus abdominalis + TX, Aphidius ervi (Aphelinus-System®) + TX, Acerophagus papaya + TX, Adalia bipunctata (Adalia-System®) + TX, Adalia bipunctata (Adaline®) + TX, Adalia bipunctata (Aphidalia®) + TX, Ageniaspis citricola + TX, Ageniaspis fuscicollis + TX, Amblyseius andersoni (Anderline®, Andersoni-System®) + TX, Amblyseius californicus (Amblyline®, Spical®) + TX, Amblyseius cucumeris (Thripex®, Bugline cucumeris®) + TX, Amblyseius fallacis (Fallacis®) + TX, Amblyseius swirskii (Bugline swirskii®, Swirskii-Mite®) + TX, Amblyseius womersleyi (WomerMite®) + TX, Amitus hesperidum + TX, Anagrus atomus + TX, Anagyrus fusciventris + TX, Anagyrus kamali + TX, Anagyrus loecki + TX, Anagyrus pseudococci (Citripar®) + TX, Anicetus benefices + TX, Anisopteromalus calandrae + TX, Anthocoris nemoralis (Anthocoris-System®) + TX, Aphelinus abdominalis (Apheline®, Aphiline®) + TX, Aphelinus asychis + TX, Aphidius colemani (Aphipar®) + TX, Aphidius ervi (Ervipar®) + TX, Aphidius gifuensis + TX, Aphidius matricariae (Aphipar- M®) + TX, Aphidoletes aphidimyza (Aphidend®) + TX, Aphidoletes aphidimyza (Aphidoline®) + TX, Aphytis lingnanensis + TX, Aphytis melinus + TX, Aprostocetus hagenowii + TX, Atheta coriaria (Staphyline®) + TX, Bombus spp. + TX, Bombus terrestris (Natupol Beehive®) + TX, Bombus terrestris (Beeline®, Tripol®) + TX, Cephalonomia stephanoderis + TX, Chilocorus nigritus + TX, Chrysoperla carnea (Chrysoline®) + TX, Chrysoperla carnea (Chrysopa®) + TX, Chrysoperla rufilabris + TX, Cirrospilus ingenuus + TX, Cirrospilus quadristriatus + TX, Citrostichus phyllocnistoides + TX, Closterocerus chamaeleon + TX, Closterocerus spp. + TX, Coccidoxenoides perminutus (Planopar®) + TX, Coccophagus cowperi + TX, Coccophagus lycimnia + TX, Cotesia flavipes + TX, Cotesia plutellae + TX, Cryptolaemus montrouzieri (Cryptobug®, Cryptoline®) + TX, Cybocephalus nipponicus + TX, Dacnusa sibirica + TX, Dacnusa sibirica (Minusa®) + TX, Diglyphus isaea (Diminex®) + TX, Delphastus catalinae (Delphastus®) + TX, Delphastus pusillus + TX, Diachasmimorpha krausii + TX, Diachasmimorpha longicaudata + TX, Diaparsis jucunda + TX, Diaphorencyrtus aligarhensis + TX, Diglyphus isaea + TX, Diglyphus isaea (Miglyphus®, Digline®) + TX, Dacnusa sibirica (DacDigline®,
Minex®) + TX, Diversinervus spp. + TX, Encarsia citrina + TX, Encarsia formosa (Encarsia max®, Encarline®, En-Strip®) + TX, Eretmocerus eremicus (Enermix®) + TX, Encarsia guadeloupae + TX, Encarsia haitiensis + TX, Episyrphus balteatus (Syrphidend®) + TX, Eretmoceris siphonini + TX, Eretmocerus californicus + TX, Eretmocerus eremicus (Ercal®, Eretline e®) + TX, Eretmocerus eremicus (Bemimix®) + TX, Eretmocerus hayati + TX, Eretmocerus mundus (Bemipar®, Eretline m®) + TX, Eretmocerus siphonini + TX, Exochomus quadripustulatus + TX, Feltiella acarisuga (Spidend®) + TX, Feltiella acarisuga (Feltiline®) + TX, Fopius arisanus + TX, Fopius ceratitivorus + TX, Formononetin (Wirless Beehome®) + TX, Franklinothrips vespiformis (Vespop®) + TX, Galendromus occidentalis + TX, Goniozus legneri + TX, Habrobracon hebetor + TX, Harmonia axyridis (HarmoBeetle®) + TX, Heterorhabditis spp. (Lawn Patrol®) + TX, Heterorhabditis bacteriophora (NemaShield HB®, Nemaseek®, Terranem-Nam®, Terranem®, Larvanem®, B-Green®, NemAttack®, Nematop®) + TX, Heterorhabditis megidis (Nemasys H®, BioNem H®, Exhibitline hm®, Larvanem-M®) + TX, Hippodamia convergens + TX, Hypoaspis aculeifer (Aculeifer-System®, Entomite-A®) + TX, Hypoaspis miles (Hypoline m®, Entomite-M®) + TX, Lbalia leucospoides + TX, Lecanoideus floccissimus + TX, Lemophagus errabundus + TX, Leptomastidea abnormis + TX, Leptomastix dactylopii (Leptopar®) + TX, Leptomastix epona + TX, Lindorus lophanthae + TX, Lipolexis oregmae + TX, Lucilia caesar (Natufly®) + TX, Lysiphlebus testaceipes + TX, Macrolophus caliginosus (Mirical-N®, Macroline c®, Mirical®) + TX, Mesoseiulus longipes + TX, Metaphycus flavus + TX, Metaphycus lounsburyi + TX, Micromus angulatus (Milacewing®) + TX, Microterys flavus + TX, Muscidifurax raptorellus and Spalangia cameroni (Biopar®) + TX, Neodryinus typhlocybae + TX, Neoseiulus californicus + TX, Neoseiulus cucumeris (THRYPEX®) + TX, Neoseiulus fallacis + TX, Nesideocoris tenuis (NesidioBug®, Nesibug®) + TX, Ophyra aenescens (Biofly®) + TX, Orius insidiosus (Thripor-I®, Oriline i®) + TX, Orius laevigatus (Thripor-L®, Oriline l®) + TX, Orius majusculus (Oriline m®) + TX, Orius strigicollis (Thripor- S®) + TX, Pauesia juniperorum + TX, Pediobius foveolatus + TX, Phasmarhabditis hermaphrodita (Nemaslug®) + TX, Phymastichus coffea + TX, Phytoseiulus macropilus + TX, Phytoseiulus persimilis (Spidex®, Phytoline p®) + TX, Podisus maculiventris (Podisus®) + TX, Pseudacteon curvatus + TX, Pseudacteon obtusus + TX, Pseudacteon tricuspis + TX, Pseudaphycus maculipennis + TX, Pseudleptomastix mexicana + TX, Psyllaephagus pilosus + TX, Psyttalia concolor (complex) + TX, Quadrastichus spp. + TX, Rhyzobius lophanthae + TX, Rodolia cardinalis + TX, Rumina decollate + TX, Semielacher petiolatus + TX, Sitobion avenae (Ervibank®) + TX, Steinernema carpocapsae (Nematac C®, Millenium®, BioNem C®, NemAttack®, Nemastar®, Capsanem®) + TX, Steinernema feltiae (NemaShield®, Nemasys F®, BioNem F, Steinernema-System®, NemAttack®, Nemaplus®, Exhibitline sf®, Scia-rid®, Entonem®) + TX, Steinernema kraussei (Nemasys L®, BioNem L®, Exhibitline srb®) + TX, Steinernema riobrave (BioVector®, BioVektor®) + TX, Steinernema scapterisci (Nematac S®) + TX, Steinernema spp. + TX, Steinernematid spp. (Guardian Nematodes®) + TX, Stethorus punctillum (Stethorus®) + TX, Tamarixia radiate + TX, Tetrastichus setifer + TX, Thripobius semiluteus + TX, Torymus sinensis + TX, Trichogramma brassicae (Tricholine b®) + TX, Trichogramma brassicae (Tricho-Strip®) + TX, Trichogramma evanescens + TX, Trichogramma minutum + TX, Trichogramma ostriniae + TX, Trichogramma platneri + TX, Trichogramma pretiosum + TX, Xanthopimpla stemmator + TX; other biologicals including: abscisic acid + TX, bioSea® + TX, Chondrostereum purpureum (Chontrol Paste®) + TX, Colletotrichum gloeosporioides (Collego®) + TX, Copper Octanoate (Cueva®) + TX,
Delta traps (Trapline d®) + TX, Erwinia amylovora (Harpin) (ProAct®, Ni-HIBIT Gold CST®) + TX, fatty acids derived from a natural by-product of extra virgin olive oil (FLIPPER®), Ferri-phosphate (Ferramol®) + TX, Funnel traps (Trapline y®) + TX, Gallex® + TX, Grower's Secret® + TX, Homo- brassonolide + TX, Iron Phosphate (Lilly Miller Worry Free Ferramol Slug & Snail Bait®) + TX, MCP hail trap (Trapline f®) + TX, Microctonus hyperodae + TX, Mycoleptodiscus terrestris (Des-X®) + TX, BioGain® + TX, Aminomite® + TX, Zenox® + TX, Pheromone trap (Thripline ams®) + TX, potassium bicarbonate (MilStop®) + TX, potassium salts of fatty acids (Sanova®) + TX, potassium silicate solution (Sil-Matrix®) + TX, potassium iodide + potassiumthiocyanate (Enzicur®) + TX, SuffOil-X® + TX, Spider venom + TX, Nosema locustae (Semaspore Organic Grasshopper Control®) + TX, Sticky traps (Trapline YF®, Rebell Amarillo®) + TX and Traps (Takitrapline y + b®) + TX; (1) antibacterial agents selected from the group of: (1.1) bacteria, examples of which are Bacillus mojavensis strain R3B (Accession No. NCAIM (P) B001389) (WO 2013/034938) from Certis USA LLC + TX; Bacillus pumilus, in particular strain BU F-33, having NRRL Accession No.50185 (CARTISSA® from BASF, EPA Reg. No.71840-19) + TX; Bacillus subtilis, in particular strain QST713/AQ713 (SERENADE OPTI or SERENADE ASO from Bayer CropScience LP, US, having NRRL Accession No. B21661, U.S. Patent No.6,060,051) + TX; Bacillus subtilis strain BU1814, (VELONDIS® PLUS, VELONDIS® FLEX and VELONDIS® EXTRA from BASF SE) + TX; Bacillus subtilis var. amyloliquefaciens strain FZB24 having Accession No. DSM 10271 (available from Novozymes as TAEGRO® or TAEGRO® ECO (EPA Registration No.70127-5)) + TX; Bacillus subtilis CX-9060 from Certis USA LLC + TX; Bacillus sp., in particular strain D747 (available as DOUBLE NICKEL® from Kumiai Chemical Industry Co., Ltd.), having Accession No. FERM BP-8234, U.S. Patent No. 7,094,592 + TX; Paenibacillus sp. strain having Accession No. NRRL B-50972 or Accession No. NRRL B-67129, WO 2016/154297 + TX; Paenibacillus polymyxa, in particular strain AC- 1 (e.g. TOPSEED® from Green Biotech Company Ltd.) + TX; Pantoea agglomerans, in particular strain E325 (Accession No. NRRL B-21856) (available as BLOOMTIME BIOLOGICAL™ FD BIOPESTICIDE from Northwest Agri Products) + TX; Pseudomonas proradix (e.g. PRORADIX® from Sourcon Padena) + TX; and (1.2) fungi, examples of which are Aureobasidium pullulans, in particular blastospores of strain DSM14940, blastospores of strain DSM 14941 or mixtures of blastospores of strains DSM14940 and DSM14941 (e.g., BOTECTOR® and BLOSSOM PROTECT® from bio-ferm, CH) + TX; Pseudozyma aphidis (as disclosed in WO2011/151819 by Yissum Research Development Company of the Hebrew University of Jerusalem) + TX; Saccharomyces cerevisiae, in particular strains CNCM No. 1-3936, CNCM No.1-3937, CNCM No.1-3938 or CNCM No.1-3939 (as disclosed in WO 2010/086790 from Lesaffre et Compagnie, FR) + TX; (2) biological fungicides selected from the group of: (2.1) bacteria, examples of which are Agrobacterium radiobacter strain K84 (e.g. GALLTROL-A® from AgBioChem, CA) + TX; Agrobacterium radiobacter strain K1026 (e.g. NOGALL™ from BASF SE) + TX; Bacillus subtilis var. amyloliquefaciens strain FZB24 having Accession No. DSM 10271 (available from Novozymes as TAEGRO® or TAEGRO® ECO (EPA Registration No. 70127-5)) + TX; Bacillus amyloliquefaciens, in particular strain D747 (available as Double Nickel™ from Kumiai Chemical Industry Co., Ltd., having accession number FERM BP-8234, US Patent No.7,094,592) + TX; Bacillus amyloliquefaciens strain F727 (also known as strain MBI110) (NRRL Accession No. B-50768, WO
2014/028521) (STARGUS® from Marrone Bio Innovations) + TX; Bacillus amyloliquefaciens strain FZB42, Accession No. DSM 23117 (available as RHIZOVITAL® from ABiTEP, DE) + TX; Bacillus amyloliquefaciens isolate B246 (e.g. AVOGREEN™ from University of Pretoria) + TX; Bacillus licheniformis, in particular strain SB3086, having Accession No. ATCC 55406, WO 2003/000051 (available as ECOGUARD® Biofungicide and GREEN RELEAF™ from Novozymes) + TX; Bacillus licheniformis FMCH001 and Bacillus subtilis FMCH002 (QUARTZO® (WG) and PRESENCE® (WP) from FMC Corporation) + TX; Bacillus methylotrophicus strain BAC-9912 (from Chinese Academy of Sciences’ Institute of Applied Ecology) + TX; Bacillus mojavensis strain R3B (Accession No. NCAIM (P) B001389) (WO 2013/034938) from Certis USA LLC + TX; Bacillus mycoides, isolate, having Accession No. B-30890 (available as BMJ TGAI® or WG and LifeGard™ from Certis USA LLC) + TX; Bacillus pumilus, in particular strain QST2808 (available as SONATA® from Bayer CropScience LP, US, having Accession No. NRRL B-30087 and described in U.S. Patent No.6,245,551) + TX; Bacillus pumilus, in particular strain GB34 (available as Yield Shield® from Bayer AG, DE) + TX; Bacillus pumilus, in particular strain BU F-33, having NRRL Accession No. 50185 (available as part of the CARTISSA product from BASF, EPA Reg. No.71840-19) + TX; Bacillus subtilis, in particular strain QST713/AQ713 (available as SERENADE OPTI or SERENADE ASO from Bayer CropScience LP, US, having NRRL Accession No. B21661 and described in U.S. Patent No. 6,060,051) + TX; Bacillus subtilis Y1336 (available as BIOBAC® WP from Bion-Tech, Taiwan, registered as a biological fungicide in Taiwan under Registration Nos.4764, 5454, 5096 and 5277) + TX; Bacillus subtilis strain MBI 600 (available as SUBTILEX from BASF SE), having Accession Number NRRL B-50595, U.S. Patent No.5,061,495 + TX; Bacillus subtilis strain GB03 (available as Kodiak® from Bayer AG, DE) + TX; Bacillus subtilis strain BU1814, (available as VELONDIS® PLUS, VELONDIS® FLEX and VELONDIS® EXTRA from BASF SE) + TX; Bacillus subtilis CX-9060 from Certis USA LLC + TX; Bacillus subtilis KTSB strain (FOLIACTIVE® from Donaghys) + TX; Bacillus subtilis IAB/BS03 (AVIV™ from STK Bio-Ag Technologies, PORTENTO® from Idai Nature) + TX; Bacillus subtilis strain Y1336 (available as BIOBAC® WP from Bion-Tech, Taiwan, registered as a biological fungicide in Taiwan under Registration Nos.4764, 5454, 5096 and 5277) + TX; Paenibacillus epiphyticus (WO 2016/020371) from BASF SE + TX; Paenibacillus polymyxa ssp. plantarum (WO 2016/020371) from BASF SE + TX; Paenibacillus sp. strain having Accession No. NRRL B-50972 or Accession No. NRRL B-67129, WO 2016/154297 + TX; Pseudomonas chlororaphis strain AFS009, having Accession No. NRRL B-50897, WO 2017/019448 (e.g., HOWLER™ and ZIO® from AgBiome Innovations, US) + TX; Pseudomonas chlororaphis, in particular strain MA342 (e.g. CEDOMON®, CERALL®, and CEDRESS® by Bioagri and Koppert) + TX; Pseudomonas fluorescens strain A506 (e.g. BLIGHTBAN® A506 by NuFarm) + TX; Pseudomonas proradix (e.g. PRORADIX® from Sourcon Padena) + TX; Streptomyces griseoviridis strain K61 (also known as Streptomyces galbus strain K61) (Accession No. DSM 7206) (MYCOSTOP® from Verdera, PREFENCE® from BioWorks, cf. Crop Protection 2006, 25, 468-475) + TX; Streptomyces lydicus strain WYEC108 (also known as Streptomyces lydicus strain WYCD108US) (ACTINO-IRON® and ACTINOVATE® from Novozymes) + TX; and (2.2) fungi, examples of which are Ampelomyces quisqualis, in particular strain AQ 10 (e.g. AQ 10® by IntrachemBio Italia) + TX; Ampelomyces quisqualis strain AQ10, having Accession No. CNCM 1-807 (e.g., AQ 10® by IntrachemBio Italia) + TX; Aspergillus flavus strain NRRL 21882 (products known as AFLA-GUARD® from Syngenta/ChemChina) + TX; Aureobasidium pullulans, in particular blastospores
of strain DSM14940 + TX; Aureobasidium pullulans, in particular blastospores of strain DSM 14941 + TX; Aureobasidium pullulans, in particular mixtures of blastospores of strains DSM14940 and DSM 14941 (e.g. Botector® by bio-ferm, CH) + TX; Chaetomium cupreum (Accession No. CABI 353812) (e.g. BIOKUPRUM™ by AgriLife) + TX; Chaetomium globosum (available as RIVADIOM® by Rivale) + TX; Cladosporium cladosporioides, strain H39, having Accession No. CBS122244, US 2010/0291039 (by Stichting Dienst Landbouwkundig Onderzoek) + TX; Coniothyrium minitans, in particular strain CON/M/91-8 (Accession No. DSM9660, e.g. Contans ® from Bayer CropScience Biologics GmbH) + TX; Cryptococcus flavescens, strain 3C (NRRL Y-50378), (B2.2.99) + TX; Dactylaria candida + TX; Dilophosphora alopecuri (available as TWIST FUNGUS®) + TX; Fusarium oxysporum, strain Fo47 (available as FUSACLEAN® by Natural Plant Protection) + TX; Gliocladium catenulatum (Synonym: Clonostachys rosea f. catenulate) strain J1446 (e.g. Prestop ® by Lallemand) + TX; Gliocladium roseum (also known as Clonostachys rosea f rosea), in particular strain 321U from Adjuvants Plus, strain ACM941 as disclosed in Xue A.G (Efficacy of Clonostachys rosea strain ACM941 and fungicide seed treatments for controlling the root tot complex of field pea, Can. J. Plant Sci.2003, 83(3): 519-524), or strain IK726 (Jensen DF, et al. Development of a biocontrol agent for plant disease control with special emphasis on the near commercial fungal antagonist Clonostachys rosea strain ’IK726’, Australasian Plant Pathol. 2007,36(2), 95-101) + TX; Lecanicillium lecanii (formerly known as Verticillium lecanii) conidia of strain KV01 (e.g. Vertalec® by Koppert/Arysta) + TX; Metschnikowia fructicola, in particular strain NRRL Y-30752, (B2.2.3) + TX; Microsphaeropsis ochracea + TX; Muscodor roseus, in particular strain A3-5 (Accession No. NRRL 30548) + TX; Penicillium steckii (DSM 27859, WO 2015/067800) from BASF SE + TX; Penicillium vermiculatum + TX; Phlebiopsis gigantea strain VRA 1992 (ROTSTOP® C from Danstar Ferment) + TX; Pichia anomala, strain WRL-076 (NRRL Y-30842), U.S. Patent No. 7,579,183 + TX; Pseudozyma flocculosa, strain PF-A22 UL (available as SPORODEX® L by Plant Products Co., CA) + TX; Saccharomyces cerevisiae, in particular strain LASO2 (from Agro-Levures et Dérivés), strain LAS117 cell walls (CEREVISANE® from Lesaffre, ROMEO® from BASF SE), strains CNCM No.1-3936, CNCM No.1-3937, CNCM No.1-3938, CNCM No.1-3939 (WO 2010/086790) from Lesaffre et Compagnie, FR + TX; Simplicillium lanosoniveum + TX; Talaromyces flavus, strain V117b + TX; Trichoderma asperelloides JM41R (Accession No. NRRL B-50759) (TRICHO PLUS® from BASF SE) + TX; Trichoderma asperellum, in particular, strain kd (e.g. T-Gro from Andermatt Biocontrol) + TX; Trichoderma asperellum, in particular strain SKT-1, having Accession No. FERM P-16510 (e.g. ECO- HOPE® from Kumiai Chemical Industry), strain T34 (e.g. T34 Biocontrol by Biocontrol Technologies S.L., ES) or strain ICC 012 from Isagro + TX; Trichoderma atroviride, in particular strain SC1 (Accession No. CBS 122089, WO 2009/116106 and U.S. Patent No.8,431,120 (from Bi-PA)), strain 77B (T77 from Andermatt Biocontrol) or strain LU132 (e.g. Sentinel from Agrimm Technologies Limited) + TX; Trichoderma atroviride, strain CNCM 1-1237 (e.g. Esquive® WP from Agrauxine, FR) + TX; Trichoderma atroviride, strain no. V08/002387 + TX; Trichoderma atroviride, strain NMI no. V08/002388 + TX; Trichoderma atroviride, strain NMI no. V08/002389 + TX; Trichoderma atroviride, strain NMI no. V08/002390 + TX; Trichoderma atroviride, strain LC52 (e.g. Tenet by Agrimm Technologies Limited) + TX; Trichoderma atroviride, strain ATCC 20476 (IMI 206040) + TX; Trichoderma atroviride, strain T11 (IMI352941/ CECT20498) + TX; Trichoderma atroviride, strain SKT-1 (FERM P-16510), JP Patent Publication (Kokai) 11-253151 A + TX; Trichoderma atroviride, strain SKT-2 (FERM P-16511), JP Patent Publication (Kokai) 11-253151 A + TX; Trichoderma atroviride, strain SKT-3 (FERM P-17021), JP Patent
Publication (Kokai) 11-253151 A + TX; Trichoderma fertile (e.g. product TrichoPlus from BASF) + TX; Trichoderma gamsii (formerly T. viride), strain ICC080 (IMI CC 392151 CABI, e.g. BioDerma by AGROBIOSOL DE MEXICO, S.A. DE C.V.) + TX; Trichoderma gamsii (formerly T. viride), strain ICC 080 (IMI CC 392151 CABI) (available as BIODERMA® by AGROBIOSOL DE MEXICO, S.A. DE C.V.) + TX; Trichoderma harmatum + TX; Trichoderma harmatum, having Accession No. ATCC 28012 + TX; Trichoderma harzianum strain T-22 (e.g. Trianum-P from Andermatt Biocontrol or Koppert) or strain Cepa SimbT5 (from Simbiose Agro) + TX; Trichoderma harzianum + TX; Trichoderma harzianum rifai T39 (e.g. Trichodex® from Makhteshim, US) + TX; Trichoderma harzianum, strain ITEM 908 (e.g. Trianum-P from Koppert) + TX; Trichoderma harzianum, strain TH35 (e.g. Root-Pro by Mycontrol) + TX; Trichoderma harzianum, strain DB 103 (available as T-GRO® 7456 by Dagutat Biolab) + TX; Trichoderma polysporum, strain IMI 206039 (e.g. Binab TF WP by BINAB Bio-Innovation AB, Sweden) + TX; Trichoderma stromaticum, having Accession No. Ts3550 (e.g. Tricovab by CEPLAC, Brazil) + TX; Trichoderma virens (also known as Gliocladium virens), in particular strain GL-21 (e.g. SoilGard by Certis, US) + TX; Trichoderma virens strain G-41, formerly known as Gliocladium virens (Accession No. ATCC 20906) (e.g., ROOTSHIELD® PLUS WP and TURFSHIELD® PLUS WP from BioWorks, US) + TX; Trichoderma viride, strain TV1(e.g. Trianum-P by Koppert) + TX; Trichoderma viride, in particular strain B35 (Pietr et al., 1993, Zesz. Nauk. A R w Szczecinie 161: 125-137) + TX; mixtures of Trichoderma asperellum strain ICC 012 (also known as Trichoderma harzianum ICC012), having Accession No. CABI CC IMI 392716 and Trichoderma gamsii (formerly T. viride) strain ICC 080, having Accession No. IMI 392151 (e.g., BIO-TAM™ from Isagro USA, Inc. or BIODERMA® by Agrobiosol de Mexico, S.A. de C.V.) + TX; Ulocladium oudemansii strain U3, having Accession No. NM 99/06216 (e.g., BOTRY-ZEN® by Botry-Zen Ltd, New Zealand and BOTRYSTOP® from BioWorks, Inc.) + TX; Verticillium albo-atrum (formerly V. dahliae), strain WCS850 having Accession No. WCS850, deposited at the Central Bureau for Fungi Cultures (e.g., DUTCH TRIG® by Tree Care Innovations) + TX; Verticillium chlamydosporium + TX; (3) biological control agents having an effect for improving plant growth and/or plant health selected from the group of: (3.1) bacteria, examples of which are Azospirillum brasilense (e.g., VIGOR® from KALO, Inc.) + TX; Azospirillum lipoferum (e.g., VERTEX-IF™ from TerraMax, Inc.) + TX; Azorhizobium caulinodans, in particular strain ZB-SK-5 + TX; Azotobacter chroococcum, in particular strain H23 + TX; Azotobacter vinelandii, in particular strain ATCC 12837 + TX; a mixture of Azotobacter vinelandii and Clostridium pasteurianum (available as INVIGORATE® from Agrinos) + TX; Bacillus amyloliquefaciens pm414 (LOLI-PEPTA® from Biofilm Crop Protection) + TX; Bacillus amyloliquefaciens SB3281 (ATCC # PTA- 7542, WO 2017/205258) + TX; Bacillus amyloliquefaciens TJ1000 (available as QUIKROOTS® from Novozymes) + TX; Bacillus amyloliquefaciens, in particular strain IN937a + TX; Bacillus amyloliquefaciens, in particular strain FZB42 (e.g. RHIZOVITAL® from ABiTEP, DE) + TX; Bacillus amyloliquefaciens BS27 (Accession No. NRRL B-5015) + TX; Bacillus cereus family member EE128 (NRRL No. B-50917) + TX; Bacillus cereus family member EE349 (NRRL No. B-50928) + TX; Bacillus cereus, in particular strain BP01 (ATCC 55675, e.g. MEPICHLOR® from Arysta Lifescience, US) + TX; Bacillus firmus, in particular strain CNMC 1-1582 (e.g. VOTIVO® from BASF SE) + TX; Bacillus mycoides BT155 (NRRL No. B-50921) + TX; Bacillus mycoides EE118 (NRRL No. B-50918) + TX; Bacillus mycoides EE141 (NRRL No. B-50916) + TX; Bacillus mycoides BT46-3 (NRRL No. B-50922)
+ TX; Bacillus pumilus, in particular strain QST2808 (Accession No. NRRL No. B-30087) + TX; Bacillus pumilus, in particular strain GB34 (e.g. YIELD SHIELD® from Bayer Crop Science, DE) + TX; Bacillus siamensis, in particular strain KCTC 13613T + TX; Bacillus subtilis, in particular strain QST713/AQ713 (having NRRL Accession No. B-21661 and described in U.S. Patent No. 6,060,051, available as SERENADE® OPTI or SERENADE® ASO from Bayer CropScience LP, US) + TX; Bacillus subtilis, in particular strain AQ30002 (Accession No. NRRL No. B-50421 and described in U.S. Patent Application No.13/330,576) + TX; Bacillus subtilis, in particular strain AQ30004 (NRRL No. B-50455 and described in U.S. Patent Application No. 13/330,576) + TX; Bacillus subtilis strain BU1814, (available as TEQUALIS® from BASF SE), Bacillus subtilis rm303 (RHIZOMAX® from Biofilm Crop Protection) + TX; Bacillus thuringiensis BT013A (NRRL No. B-50924) also known as Bacillus thuringiensis 4Q7 + TX; a mixture of Bacillus licheniformis FMCH001 and Bacillus subtilis FMCH002 (available as QUARTZO® (WG), PRESENCE® (WP) from FMC Corporation) + TX; Bacillus subtilis, in particular strain MBI 600 (e.g. SUBTILEX® from BASF SE) + TX; Bacillus tequilensis, in particular strain NII-0943 + TX; Bradyrhizobium japonicum (e.g. OPTIMIZE® from Novozymes) + TX; Delftia acidovorans, in particular strain RAY209 (e.g. BIOBOOST® from Brett Young Seeds) + TX; Mesorhizobium cicer (e.g., NODULATOR from BASF SE) + TX; Lactobacillus sp. (e.g. LACTOPLANT® from LactoPAFI) + TX; Rhizobium leguminosarium biovar viciae (e.g., NODULATOR from BASF SE) + TX; Pseudomonas proradix (e.g. PRORADIX® from Sourcon Padena) + TX; Pseudomonas aeruginosa, in particular strain PN1 + TX; Rhizobium leguminosarum, in particular bv. viceae strain Z25 (Accession No. CECT 4585) + TX; Paenibacillus polymyxa, in particular strain AC-1 (e.g. TOPSEED® from Green Biotech Company Ltd.) + TX; Serratia marcescens, in particular strain SRM (Accession No. MTCC 8708) + TX; Sinorhizobium meliloti strain NRG-185-1 (NITRAGIN® GOLD from Bayer CropScience) + TX; Thiobacillus sp. (e.g. CROPAID® from Cropaid Ltd UK) + TX; and (3.2) fungi, examples of which are Purpureocillium lilacinum (previously known as Paecilomyces lilacinus) strain 251 (AGAL 89/030550, e.g. BioAct from Bayer CropScience Biologics GmbH) + TX; Penicillium bilaii, strain ATCC 22348 (e.g. JumpStart® from Acceleron BioAg), Talaromyces flavus, strain V117b + TX; Trichoderma atroviride strain CNCM 1-1237 (e.g. Esquive® WP from Agrauxine, FR), Trichoderma viride, e.g. strain B35 (Pietr et al., 1993, Zesz. Nauk. A R w Szczecinie 161: 125-137) + TX; Trichoderma atroviride strain LC52 (also known as Trichoderma atroviride strain LU132, e.g. Sentinel® from Agrimm Technologies Limited) + TX; Trichoderma atroviride strain SC1 described in WO2009/116106) + TX; Trichoderma asperellum strain kd (e.g. T-Gro from Andermatt Biocontrol) + TX; Trichoderma asperellum strain (Eco-T from Plant Health Products, ZA) + TX, Trichoderma harzianum strain T-22 (e.g. Trianum-P from Andermatt Biocontrol or Koppert) + TX; Myrothecium verrucaria strain AARC-0255 (e.g. DiTera™ from Valent Biosciences) + TX; Penicillium bilaii strain ATCC ATCC20851 + TX; Pythium oligandrum strain M1 (ATCC 38472, e.g. Polyversum from Bioprepraty, CZ) + TX; Trichoderma virens strain GL-21 (e.g. SoilGard® from Certis, USA) + TX; Verticillium albo-atrum (formerly V. dahliae) strain WCS850 (CBS 276.92, e.g. Dutch Trig from Tree Care Innovations) + TX; Trichoderma atroviride, in particular strain no. V08/002387, strain no. NMI No. V08/002388, strain no. NMI No. V08/002389, strain no. NMI No. V08/002390 + TX; Trichoderma harzianum strain ITEM 908, Trichoderma harzianum, strain TSTh20 + TX; Trichoderma harzianum strain 1295-22 + TX; Pythium oligandrum strain DV74 + TX; Rhizopogon amylopogon (e.g. Myco-Sol from Agri-Enterprise, LLC,
formerly Helena Chemical Company) + TX; Rhizopogon fulvigleba (e.g. Myco-Sol from Agri-Enterprise, LLC, formerly Helena Chemical Company) + TX; Trichoderma virens strain GI-3 + TX; (4) insecticidally active biological control agents selected from (4.1) bacteria, examples of which are Agrobacterium radiobacter strain K84 (Galltrol from AgBiochem Inc.) + TX; Bacillus amyloliquefaciens, in particular strain PTS-4838 (e.g. AVEO from Valent Biosciences, US) + TX; Bacillus firmus, in particular strain CNMC 1-1582 (e.g. VOTIVO® from BASF SE) + TX; Bacillus mycoides, isolate J. (e.g. BmJ from Certis USA LLC.) + TX; Bacillus sphaericus, in particular Serotype H5a5b strain 2362 (strain ABTS-1743) (e.g. VECTOLEX® from Valent BioSciences, US) + TX; Bacillus thuringiensis subsp. aizawai, in particular strain ABTS-1857 (SD-1372, e.g. XENTARI® from Valent BioSciences) + TX; Bacillus thuringiensis subsp. aizawai, in particular serotype H-7 (e.g. FLORBAC® WG from Valent BioSciences, US) + TX; Bacillus thuringiensis israelensis strain BMP 144 (e.g. AQUABAC® by Becker Microbial Products IL) + TX; Bacillus thuringiensis subsp. israelensis (serotype H-14) strain AM65-52 (Accession No. ATCC 1276) (e.g. VECTOBAC® by Valent BioSciences, US) + TX; Bacillus thuringiensis subsp. aizawai strain GC-91 + TX; Bacillus thuringiensis var. Colmeri (e.g. TIANBAOBTC by Changzhou Jianghai Chemical Factory) + TX; Bacillus thuringiensis var. japonensis strain Buibui + TX; Bacillus thuringiensis subsp. kurstaki strain BMP 123 (from Becker Microbial Products, IL, BARITONE from Bayer CropScience) + TX; Bacillus thuringiensis subsp. kurstaki strain HD-1 (e.g. DIPEL® ES from Valent BioSciences, US) + TX; Bacillus thuringiensis var. kurstaki strain EVB-113-19 (e.g., BIOPROTEC® from AEF Global) + TX; Bacillus thuringiensis subsp. kurstaki strain ABTS 351 + TX; Bacillus thuringiensis subsp. kurstaki strain PB 54 + TX; Bacillus thuringiensis subsp. kurstaki strain SA 11 (JAVELIN from Certis, US) + TX; Bacillus thuringiensis subsp. kurstaki strain SA 12 (THURICIDE from Certis, US) + TX; Bacillus thuringiensis subsp. kurstaki strain EG 2348 (LEPINOX® from Certis, US) + TX; Bacillus thuringiensis subsp. kurstaki strain EG 7841 (CRYMAX® from Certis, US) + TX; Bacillus thuringiensis subsp. tenebrionis strain NB 176 (SD-5428, e.g. NOVODOR® FC from BioFa DE) + TX; Brevibacillus laterosporus (LATERAL® from Ecolibrium Biologicals) + TX; Burkholderia spp., in particular Burkholderia rinojensis strain A396 (also known as Burkholderia rinojensis strain MBI 305) (Accession No. NRRL B-50319); WO 2011/106491 and WO 2013/032693; e.g. MBI206 TGAI and ZELTO® from Marrone Bio Innovations) + TX; Chromobacterium subtsugae, in particular strain PRAA4-1T (e.g. MBI-203; e.g. GRANDEVO® from Marrone Bio Innovations) + TX; Lecanicillium muscarium Ve6 (MYCOTAL from Koppert) + TX; Paenibacillus popilliae (formerly Bacillus popilliae + TX; e.g. MILKY SPORE POWDER™ or MILKY SPORE GRANULAR™ from St. Gabriel Laboratories) + TX; Pasteuria nishizawae strain Pn1 (CLARIVA from Syngenta/ChemChina) + TX;Serratia entomophila (e.g. INVADE® by Wrightson Seeds) + TX; Serratia marcescens, in particular strain SRM (Accession No. MTCC 8708) + TX;Trichoderma asperellum (TRICHODERMAX from Novozymes) + TX; Wolbachia pipientis ZAP strain (e.g., ZAP MALES® from MosquitoMate) + TX; and (4.2) fungi, examples of which are Beauveria bassiana strain ATCC 74040 (e.g. NATURALIS® from Intrachem Bio Italia) + TX; Beauveria bassiana strain GHA (Accession No. ATCC74250, e.g. BOTANIGUARD® ES and MYCONTROL-O® from Laverlam International Corporation) + TX; Beauveria bassiana strain ATP02 (Accession No. DSM 24665) + TX; Isaria fumosorosea (previously known as Paecilomyces fumosoroseus) strain Apopka 97 (PREFERAL® from SePRO) + TX; Metarhizium anisopliae 3213-1 (deposited under NRRL accession number 67074 disclosed in WO 2017/066094;
Pioneer Hi-Bred International) + TX; Metarhizium robertsii 15013-1 (deposited under NRRL accession number 67073) + TX; Metarhizium robertsii 23013-3 (deposited under NRRL accession number 67075) + TX; Paecilomyces lilacinus strain 251 (MELOCON® from Certis, US) + TX; Zoophtora radicans + TX; (5) Viruses selected from the group consisting of Adoxophyes orana (summer fruit tortrix) granulosis virus (GV) + TX; Cydia pomonella (codling moth) granulosis virus (GV) + TX; Helicoverpa armigera (cotton bollworm) nuclear polyhedrosis virus (NPV) + TX; Spodoptera exigua (beet armyworm) mNPV + TX; Spodoptera frugiperda (fall armyworm) mNPV + TX; Spodoptera littoralis (African cotton leafworm) NPV + TX; (6) Bacteria and fungi which can be added as ’inoculant’ to plants or plant parts or plant organs and which, by virtue of their particular properties, promote plant growth and plant health selected from Agrobacterium spp. + TX; Azorhizobium caulinodans + TX; Azospirillum spp. + TX; Azotobacter spp. + TX; Bradyrhizobium spp. + TX; Burkholderia spp., in particular Burkholderia cepacia (formerly known as Pseudomonas cepacia) + TX; Gigaspora spp., or Gigaspora monosporum + TX; Glomus spp. + TX; Laccaria spp. + TX; LactoBacillus buchneri + TX; Paraglomus spp. + TX; Pisolithus tinctorus + TX; Pseudomonas spp. + TX; Rhizobium spp., in particular Rhizobium trifolii + TX; Rhizopogon spp. + TX; Scleroderma spp. + TX; Suillus spp. + TX; Streptomyces spp. + TX; (7) Plant extracts and products formed by microorganisms including proteins and secondary metabolites which can be used as biological control agents, selected from Allium sativum (NEMGUARD from Eco-Spray; BRALIC from ADAMA) + TX; Armour-Zen + TX; Artemisia absinthium + TX; Azadirachtin (e.g. AZATIN XL from Certis, US) + TX; Biokeeper WP + TX; Brassicaceae extract, in particular oilseed rape powder or mustard powder + TX; Cassia nigricans + TX; Celastrus angulatus + TX; Chenopodium anthelminticum + TX; Chitin + TX; Dryopteris filix-mas + TX; Equisetum arvense + TX; Fortune Aza + TX; Fungastop + TX; Chenopodium quinoa saponin extract from quinoa seeds (e.g. Heads Up® (Saponins of Quinoa) from Heads Up plant Protectants, CA) + TX; naturally occurring Blad polypeptide extracted from Lupin seeds (PROBLAD® from Certis EU) + TX; naturally occurring Blad polypeptide extracted from Lupin seeds (FRACTURE® from FMC) + TX; Pyrethrum/Pyrethrins + TX; Quassia amara + TX; Quercus + TX; Quillaja extract (QL AGRI 35 from BASF) + TX; Reynoutria sachalinensis extract (REGALLIA®, REGALIA® MAXX from Marrone Bio) + TX; "Requiem ™ Insecticide" + TX; Rotenone + TX; ryania/ryanodine + TX; Symphytum officinale + TX; Tanacetum vulgare + TX; Thymol + TX; Thymol mixed with Geraniol (CEDROZ from Eden Research) + TX; Thymol mixed with Geraniol and Eugenol (MEVALONE® from Eden Research) + TX; Triact 70 + TX; TriCon + TX; Tropaeulum majus + TX; Melaleuca alternifolia extract (TIMOREX GOLD from STK) + TX; Urtica dioica + TX; Veratrin + TX; and Viscum album + TX; and a safener, such as benoxacor + TX, cloquintocet (including cloquintocet-mexyl) + TX, cyprosulfamide + TX, dichlormid + TX, fenchlorazole (including fenchlorazole-ethyl) + TX, fenclorim + TX, fluxofenim + TX, furilazole + TX, isoxadifen (including isoxadifen-ethyl) + TX, mefenpyr (including mefenpyr-diethyl) + TX, metcamifen + TX and oxabetrinil + TX. In addition, the compositions of the invention may also be applied with one or more systemically acquired resistance inducers (“SAR” inducer). SAR inducers are known and described in, for example, United States Patent No. US 6,919,298 and include, for example, salicylates and the commercial SAR inducer acibenzolar-S-methyl.
The compounds of formula (I) as defined in the present invention are normally used in the form of compositions and can be applied to the crop area or plant to be treated, simultaneously or in succession with further compounds. These further compounds can be e.g. fertilizers or micronutrient donors or other preparations, which influence the growth of plants. They can also be selective herbicides or non- selective herbicides as well as insecticides, fungicides, bactericides, nematicides, molluscicides or mixtures of several of these preparations, if desired together with further carriers, surfactants or application promoting adjuvants customarily employed in the art of formulation. The compounds of formula (I) as defined in the present invention may be used in the form of (fungicidal) compositions for controlling or protecting against phytopathogenic microorganisms, comprising as active ingredient at least one compound of formula (I) as defined in any one of embodiments 1 to 43 or of at least one preferred individual compound as above-defined, in free form or in agrochemically usable salt form, and at least one of the above-mentioned adjuvants. The invention therefore provides a composition, preferably a fungicidal composition, comprising at least one compound of formula (I) as defined in the present invention, an agriculturally acceptable carrier and optionally an adjuvant. An agricultural acceptable carrier is for example a carrier that is suitable for agricultural use. Agricultural carriers are well known in the art. Preferably said composition may comprise at least one or more pesticidally active compounds, for example an additional fungicidal active ingredient in addition to the compound of formula (I) as defined in the present invention. A further aspect of invention is related to a method of controlling or preventing an infestation of plants, e.g. useful plants such as crop plants, propagation material thereof, e.g. seeds, harvested crops, e.g. harvested food crops, or of non-living materials by phytopathogenic or spoilage microorganisms or organisms potentially harmful to man, especially fungal organisms, which comprises the application of a compound of formula (I) as defined in the present invention or of a preferred individual compound as above-defined as active ingredient to the plants, to parts of the plants or to the locus thereof, to the propagation material thereof, or to any part of the non-living materials. Controlling or preventing means reducing infestation by insects or by phytopathogenic or spoilage microorganisms or organisms potentially harmful to man, especially fungal organisms, to such a level that an improvement is demonstrated. A preferred method of controlling or preventing an infestation of crop plants by phytopathogenic microorganisms, especially fungal organisms, which comprises the application of a compound of formula (I) as defined in the present invention, or an agrochemical composition which contains at least one of said compounds, is foliar application. The frequency of application and the rate of application will depend on the risk of infestation by the corresponding pathogen or insect. However, the compounds of formula (I) as defined in the present invention can also penetrate the plant through the roots via the soil (systemic action) by drenching the locus of the plant with a liquid formulation, or by applying the compounds in solid form to the soil, e.g. in granular form (soil application). In crops of water rice such granulates can
be applied to the flooded rice field. The compounds of formula (I) as defined in any the present invention may also be applied to seeds (coating) by impregnating the seeds or tubers either with a liquid formulation of the fungicide or coating them with a solid formulation. A formulation, e.g. a composition containing the compound of formula (I) as defined in the present invention, and, if desired, a solid or liquid adjuvant or monomers for encapsulating the compound of formula (I) as defined in the present invention, may be prepared in a known manner, typically by intimately mixing and/or grinding the compound with extenders, for example solvents, solid carriers and, optionally, surface active compounds (surfactants). The application methods for the compositions, that is the methods of controlling pathogens of the abovementioned type, such as spraying, atomizing, dusting, brushing on, dressing, scattering or pouring - which are to be selected to suit the intended aims of the prevailing circumstances - and the use of the compositions for controlling pathogens of the abovementioned type are other subjects of the invention. Typical rates of concentration are between 0.1 and 1000 ppm, preferably between 0.1 and 500 ppm, of active ingredient. The rate of application per hectare is preferably 1g to 2000 g of active ingredient per hectare, more preferably 10 to 1000 g/ha, most preferably 10 to 600 g/ha. When used as seed drenching agent, convenient dosages are from 10mg to 1g of active substance per kg of seeds. When the combinations of the present invention are used for treating seed, rates of 0.001 to 50 g of a compound of formula (I) per kg of seed, preferably from 0.01 to 10g per kg of seed are generally sufficient. Suitably, a composition comprising a compound of formula (I) as defined in the present invention according to the present invention is applied either preventative, meaning prior to disease development or curative, meaning after disease development. The compositions of the invention may be employed in any conventional form, for example in the form of a twin pack, a powder for dry seed treatment (DS), an emulsion for seed treatment (ES), a flowable concentrate for seed treatment (FS), a solution for seed treatment (LS), a water dispersible powder for seed treatment (WS), a capsule suspension for seed treatment (CF), a gel for seed treatment (GF), an emulsion concentrate (EC), a suspension concentrate (SC), a suspo-emulsion (SE), a capsule suspension (CS), a water dispersible granule (WG), an emulsifiable granule (EG), an emulsion, water in oil (EO), an emulsion, oil in water (EW), a micro-emulsion (ME), an oil dispersion (OD), an oil miscible flowable (OF), an oil miscible liquid (OL), a soluble concentrate (SL), an ultra-low volume suspension (SU), an ultra-low volume liquid (UL), a technical concentrate (TK), a dispersible concentrate (DC), a wettable powder (WP) or any technically feasible formulation in combination with agriculturally acceptable adjuvants. Such compositions may be produced in conventional manner, e.g. by mixing the active ingredients with appropriate formulation inerts (diluents, solvents, fillers and optionally other formulating ingredients such as surfactants, biocides, anti-freeze, stickers, thickeners and compounds that provide adjuvancy
effects). Also conventional slow release formulations may be employed where long lasting efficacy is intended. Particularly formulations to be applied in spraying forms, such as water dispersible concentrates (e.g. EC, SC, DC, OD, SE, EW, EO and the like), wettable powders and granules, may contain surfactants such as wetting and dispersing agents and other compounds that provide adjuvancy effects, e.g. the ondensation product of formaldehyde with naphthalene sulphonate, an alkylarylsulphonate, a lignin sulphonate, a fatty alkyl sulphate, and ethoxylated alkylphenol and an ethoxylated fatty alcohol. A seed dressing formulation is applied in a manner known per se to the seeds employing the combination of the invention and a diluent in suitable seed dressing formulation form, e.g. as an aqueous suspension or in a dry powder form having good adherence to the seeds. Such seed dressing formulations are known in the art. Seed dressing formulations may contain the single active ingredients or the combination of active ingredients in encapsulated form, e.g. as slow release capsules or microcapsules. In general, the formulations include from 0.01 to 90% by weight of active agent, from 0 to 20% agriculturally acceptable surfactant and 10 to 99.99% solid or liquid formulation inerts and adjuvant(s), the active agent consisting of at least the compound of formula (I) as defined in the present invention together with component (B) and (C), and optionally other active agents, particularly microbiocides or conservatives or the like. Concentrated forms of compositions generally contain in between about 2 and 80%, preferably between about 5 and 70% by weight of active agent. Application forms of formulation may for example contain from 0.01 to 20% by weight, preferably from 0.01 to 5% by weight of active agent. Whereas commercial products will preferably be formulated as concentrates, the end user will normally employ diluted formulations. Whereas it is preferred to formulate commercial products as concentrates, the end user will normally use dilute formulations. EXAMPLES The Examples which follow serve to illustrate the invention. Certain compounds of the invention can be distinguished from known compounds by virtue of greater efficacy at low application rates, which can be verified by the person skilled in the art using the experimental procedures outlined in the Examples,. Compounds of Formula (I) may possess any number of benefits including, inter alia, advantageous levels of biological activity for protecting plants against diseases that are caused by fungi or superior properties for use as agrochemical active ingredients (for example, greater biological activity, an advantageous spectrum of activity, an increased safety profile (including improved crop tolerance), improved physico-chemical properties, or increased biodegradability). Throughout this description, temperatures are given in degrees Celsius (°C) and “MP” means melting point. LC/MS means Liquid Chromatography Mass Spectrometry and the description of the apparatus and the methods are described below.
Formulation Examples Wettable powders a) b) c) active ingredient [compound of formula (I)] 25 % 50 % 75 % sodium lignosulfonate 5 % 5 % - sodium lauryl sulfate 3 % - 5 % sodium diisobutylnaphthalenesulfonate - 6 % 10 % phenol polyethylene glycol ether - 2 % - (7-8 mol of ethylene oxide) highly dispersed silicic acid 5 % 10 % 10 % Kaolin 62 % 27 % - The active ingredient is thoroughly mixed with the adjuvants and the mixture is thoroughly ground in a suitable mill, affording wettable powders that can be diluted with water to give suspensions of the desired concentration. Powders for dry seed treatment a) b) c) active ingredient [compound of formula (I)] 25 % 50 % 75 % light mineral oil 5 % 5 % 5 % highly dispersed silicic acid 5 % 5 % - Kaolin 65 % 40 % - Talcum - 20% The active ingredient is thoroughly mixed with the adjuvants and the mixture is thoroughly ground in a suitable mill, affording powders that can be used directly for seed treatment. Emulsifiable concentrate active ingredient [compound of formula (I)] 10 % octylphenol polyethylene glycol ether 3 % (4-5 mol of ethylene oxide) calcium dodecylbenzenesulfonate 3 % castor oil polyglycol ether (35 mol of ethylene oxide) 4 % Cyclohexanone 30 % xylene mixture 50 % Emulsions of any required dilution, which can be used in plant protection, can be obtained from this concentrate by dilution with water.
Dusts a) b) c) Active ingredient [compound of formula (I)] 5 % 6 % 4 % talcum 95 % - - Kaolin - 94 % - mineral filler - - 96 % Ready-for-use dusts are obtained by mixing the active ingredient with the carrier and grinding the mixture in a suitable mill. Such powders can also be used for dry dressings for seed. Extruder granules Active ingredient [compound of formula (I)] 15 % sodium lignosulfonate 2 % carboxymethylcellulose 1 % Kaolin 82 % The active ingredient is mixed and ground with the adjuvants, and the mixture is moistened with water. The mixture is extruded and then dried in a stream of air. Coated granules Active ingredient [compound of formula (I)] 8 % polyethylene glycol (mol. wt.200) 3 % Kaolin 89 % The finely ground active ingredient is uniformly applied, in a mixer, to the kaolin moistened with polyethylene glycol. Non-dusty coated granules are obtained in this manner. Suspension concentrate active ingredient [compound of formula (I)] 40 % propylene glycol 10 % nonylphenol polyethylene glycol ether (15 mol of ethylene oxide) 6 % Sodium lignosulfonate 10 % carboxymethylcellulose 1 % silicone oil (in the form of a 75 % emulsion in water) 1 % Water 32 % The finely ground active ingredient is intimately mixed with the adjuvants, giving a suspension concentrate from which suspensions of any desired dilution can be obtained by dilution with water. Using such dilutions, living plants as well as plant propagation material can be treated and protected against infestation by microorganisms, by spraying, pouring or immersion. Flowable concentrate for seed treatment active ingredient [compound of formula (I)] 40 %
propylene glycol 5 % copolymer butanol PO/EO 2 % tristyrenephenole with 10-20 moles EO 2 % 1,2-benzisothiazolin-3-one (in the form of a 20% solution in water) 0.5 % monoazo-pigment calcium salt 5 % Silicone oil (in the form of a 75 % emulsion in water) 0.2 % Water 45.3 % The finely ground active ingredient is intimately mixed with the adjuvants, giving a suspension concentrate from which suspensions of any desired dilution can be obtained by dilution with water. Using such dilutions, living plants as well as plant propagation material can be treated and protected against infestation by microorganisms, by spraying, pouring or immersion. Slow Release Capsule Suspension 28 parts of a combination of the compound of formula (I) are mixed with 2 parts of an aromatic solvent and 7 parts of toluene diisocyanate/polymethylene-polyphenylisocyanate-mixture (8:1). This mixture is emulsified in a mixture of 1.2 parts of polyvinylalcohol, 0.05 parts of a defoamer and 51.6 parts of water until the desired particle size is achieved. To this emulsion a mixture of 2.8 parts 1,6-diaminohexane in 5.3 parts of water is added. The mixture is agitated until the polymerization reaction is completed. The obtained capsule suspension is stabilized by adding 0.25 parts of a thickener and 3 parts of a dispersing agent. The capsule suspension formulation contains 28% of the active ingredients. The medium capsule diameter is 8-15 microns. The resulting formulation is applied to seeds as an aqueous suspension in an apparatus suitable for that purpose. Analytical Methods Method A: Equipment: Shimadzu LCMS 2020 Mass Spectrometer; Column: HALO C182.7 µm, 3.0 mm × 30 mm; Mobile Phase: MeCN (with either 0.05% HCOOH or 0.05% TFA) - Water (with either 0.05% HCOOH or 0.05% TFA); Gradient: MeCN from 5% to 95% over 1.4 min, hold 0.6 min, total run time is 2.5 min; Flow rate: 1.8 mL/min; Column temperature: 50
°C; Wavelength: 214 and 254 nm PDA. Method B: Spectra were recorded on a Mass Spectrometer from Waters (Acquity QDa Mass Spectrometer) equipped with an electrospray source (Polarity: Positive and Negative Polarity Switch), Capillary: 0.8 kV, Cone range: 25 V, Extractor: V (No extractor voltage for QDa detector) Source Temperature: 120°C, Desolvation Temperature: 600°C, Cone Gas Flow: 50 L/h, Desolvation Gas Flow: 1000 L/h, Mass range: 110 to 850 Da) and an Acquity UPLC from Waters: Quaternary solvent manager, heated column compartment , diode-array detector. Column: Acquity UPLC HSS T3 C18, 1.8 µm, 30 x 2.1 mm, Temp: 40 °C, DAD Wavelength range (nm): 200 to 400, Solvent Gradient: A = water + 5% Acetonitrile + 0.1 %
HCOOH, B= Acetonitrile + 0.05 % HCOOH: gradient: 0 min 10% B; 0.-0.2 min 10-50% B; 0.2-0.6 min 50-100% B; 0.6-1.3 min 100% B; 1.3-1.4 min 100-10% B; 1.4-1.6 min 10% B; Flow (mL/min) 0.6. Method C: Spectra were recorded on a Mass Spectrometer from Waters Corporation (SQD, SQDII or QDA Single quadrupole mass spectrometer) equipped with an electrospray source (Polarity: positive and negative ions), Capillary: 0.8-3.00 kV, Cone: 5-30 V, Source Temperature: 120-150°C, Desolvation Temperature: 350-600°C, Cone Gas Flow: 50-150 l/h, Desolvation Gas Flow: 650-1000 l/h, Mass range: 110 to 950 Da and an Acquity UPLC from Waters Corporation: Binary pump, heated column compartment, diode- array detector and ELSD. Column: Waters UPLC HSS T3, 1.8 µm, 30 x 2.1 mm, Temp: 60 °C, DAD Wavelength range (nm): 210 to 400, Runtime: 1.5 min; Solvents: A = water + 5% MeOH + 0.05 % HCOOH, B= Acetonitrile + 0.05 % HCOOH; Flow (mL/min) 0.85, Gradient: 10% B isocratic for 0.2 min, then 10-100% B in 1.0 min, 100% B isocratic for 0.2min, 100-10% B in 0.05min, 10% B isocratic for 0.05 min Method D: Spectra were recorded on a Mass Spectrometer from Agilent (Single quad mass spectrometer) equipped with a Multimode- Electron Spray and APCI (Polarity: positive and negative ions), Capillary: 4.00 kV, Corona Current 4.0 µA, Charging Voltage, 2.00 kV, Nitrogen Gas Flow: 12.0 L/min, Nebulizer Pressure: 40 psig, Mass range: 100 to 1000 m/z), dry gas temperature 250 °C, Vaporizer temperature 200 °C and an UPLC from Waters: quaternary pump, heated column compartment, Variable wave length detector. Column: X-Bridge BEH C18, 2.5 µm (2.1 * 50 mm), column Temp: Ambient, Wavelength (nm): 215 nm, Gradient: A = 0.05% TFA in water, B = 0.05% TFA in Acetonitrile. Gradient: time/%B: 0/5, 1/5, 5/70, 7/95, 8.5/95, 8.6/5, 10/5; Flow rate: 0.6 mL/min. The below Table A gathers for compounds of formula (I): - LC/MS data, such as retention time (RT), [M+H]
+, - the type of methods, and/or - melting point (MP). Table A: Entry IUPAC name Chemical structure RT [M+H] Method MP (min) (measured) (°C) 1 methyl N-[5-[6-[(4-fluoro-3-methoxy- 1.10 494 A 174- phenyl)-methyl-carbamoyl]-4- 177 (methoxymethyl)benzimidazol-1-yl]-2- pyridyl]carbamate
Entry IUPAC name Chemical structure RT [M+H] Method MP (min) (measured) (°C) 2 methyl N-[5-[6-[2-cyanoethyl-(4-fluoro- 1.43 503 A 142- 3-methoxy-phenyl)carbamoyl]-4-methyl- 146 benzimidazol-1-yl]-2-pyridyl]carbamate 3 methyl N-[5-[4-ethyl-6-[(4-fluoro-3- 1.28 478 A 193- methoxy-phenyl)-methyl- 194 carbamoyl]benzimidazol-1-yl]-2- pyridyl]carbamate 4 methyl N-[5-[6-[(4-fluoro-3-methoxy- 1.12 524 A 98- phenyl)-(methoxymethyl)carbamoyl]-4- 100 (methoxymethyl)benzimidazol-1-yl]-2- pyridyl]carbamate 5 methyl N-[5-[6-[ethyl-(4-fluoro-3- 1.17 508 A 185- methoxy-phenyl)carbamoyl]-4- 187 (methoxymethyl)benzimidazol-1-yl]-2- pyridyl]carbamate 6 methyl N-[5-[4-ethyl-6-[(4-fluoro-3- 1.18 508 A 140- methoxy-phenyl)- 145 (methoxymethyl)carbamoyl]benzimidaz ol-1-yl]-2-pyridyl]carbamate 7 methyl N-[5-[6-[cyanomethyl-(4- 1.13 459 A 144- fluorophenyl)carbamoyl]-4-methyl- 145 benzimidazol-1-yl]-2-pyridyl]carbamate 8 methyl N-[5-[6-[cyanomethyl-(4-fluoro- 1.13 489 A 101- 3-methoxy-phenyl)carbamoyl]-4-methyl- 103 benzimidazol-1-yl]-2-pyridyl]carbamate 9 methyl N-[5-[6-[(4-cyano-3-methoxy- 1.16 471 A 255- phenyl)-methyl-carbamoyl]-4-methyl- 257 benzimidazol-1-yl]-2-pyridyl]carbamate
Entry IUPAC name Chemical structure RT [M+H] Method MP (min) (measured) (°C) 10 methyl N-[5-[6-[(4-cyano-3-fluoro- 1.42 459 A 140- phenyl)-methyl-carbamoyl]-4-methyl- 142 benzimidazol-1-yl]-2-pyridyl]carbamate 11 methyl N-[5-[6-[(4-fluoro-3-methoxy- 1.00 465 A 214- phenyl)-methyl-carbamoyl]-4-methyl- 217 imidazo[4,5-c]pyridin-1-yl]-2- pyridyl]carbamate 12 methyl N-[5-[5-[(4-fluoro-3-methoxy- 1.32 451 A 179- phenyl)-methyl-carbamoyl]imidazo[4,5- 181 b]pyridin-3-yl]-2-pyridyl]carbamate 13 methyl N-[5-[6-[(4-fluoro-3-methoxy- 1.14 508 A 216- phenyl)-(2-methoxyethyl)carbamoyl]-4- 217 methyl-benzimidazol-1-yl]-2- pyridyl]carbamate 14 3-(6-acetamido-3-pyridyl)-N-(4-fluoro-3- 1.07 448 A 118- methoxy-phenyl)-N,7-dimethyl- 119 benzimidazole-5-carboxamide 15 N-(4-fluoro-3-methoxy-phenyl)-N,7- 1.05 463 A 218- dimethyl-3-[6-(methylcarbamoylamino)- 221 3-pyridyl]benzimidazole-5-carboxamide 16 ethyl N-[5-[6-[(4-fluoro-3-methoxy- 1.19 478 A 103- phenyl)-methyl-carbamoyl]-4-methyl- 104 benzimidazol-1-yl]-2-pyridyl]carbamate 17 methyl N-[5-[4-ethyl-6-[(4-fluorophenyl)- 2.39 478 A 98- (methoxymethyl)carbamoyl]benzimidaz 101 ol-1-yl]-2-pyridyl]carbamate
Entry IUPAC name Chemical structure RT [M+H] Method MP (min) (measured) (°C) 18 3-[6-(cyclopropanecarbonylamino)-3- 1.16 474 A 109- pyridyl]-N-(4-fluoro-3-methoxy-phenyl)- 110 N,7-dimethyl-benzimidazole-5- carboxamide 19 methyl N-[5-[6-[(2-methoxy-4-pyridyl)- 1.05 447 A 113- methyl-carbamoyl]-4-methyl- 115 benzimidazol-1-yl]-2-pyridyl]carbamate 20 methyl N-[5-[6-(6-fluoro-4-methyl-2,3- 1.22 475 A 140- dihydroquinoxaline-1-carbonyl)-4- 142 methyl-benzimidazol-1-yl]-2- pyridyl]carbamate 21 methyl N-[5-[5-[(4-fluorophenyl)-methyl- 1.11 435 A 233- carbamoyl]-7-methyl-imidazo[4,5- 235 b]pyridin-3-yl]-2-pyridyl]carbamate 22 methyl N-[5-[6-[(4-fluorophenyl)-methyl- 0.82 420 C carbamoyl]benzimidazol-1-yl]-2- pyridyl]carbamate 23 methyl N-[5-[6-[(4-fluoro-3-methoxy- 1.02 494 B 135- phenyl)-(methoxymethyl)carbamoyl]-4- 140 methyl-benzimidazol-1-yl]-2- pyridyl]carbamate 24 methyl N-[5-[6-[(4-fluoro-3-methoxy- 3.20 451 D 203- phenyl)-methyl-carbamoyl]imidazo[4,5- 208 c]pyridin-1-yl]-2-pyridyl]carbamate 25 methyl N-[5-[6-[(4-fluorophenyl)-methyl- 0.85 434 C carbamoyl]-4-methyl-benzimidazol-1- yl]-2-pyridyl]carbamate
Entry IUPAC name Chemical structure RT [M+H] Method MP (min) (measured) (°C) 26 methyl N-[5-[6-[(4-fluoro-3-methoxy- 0.86 464 C 150- phenyl)-methyl-carbamoyl]-4-methyl- 155 benzimidazol-1-yl]-2-pyridyl]carbamate 27 methyl N-[5-[6-[(4-fluorophenyl)- 1.09 494 A 154- (methoxymethyl)carbamoyl]-4- 158 (methoxymethyl)benzimidazol-1-yl]-2- pyridyl]carbamate 28 methyl N-[5-[6-[(4-fluorophenyl)-(2- 1.14 478 A 101- methoxyethyl)carbamoyl]-4-methyl- 103 benzimidazol-1-yl]-2-pyridyl]carbamate 29 methyl N-[5-[6-[2-cyanoethyl-(4- 1.09 473 A 115- fluorophenyl)carbamoyl]-4-methyl- 118 benzimidazol-1-yl]-2-pyridyl]carbamate 30 3-(6-acetamido-3-pyridyl)-N-(4- 1.05 418 A 97-98 fluorophenyl)-N,7-dimethyl- benzimidazole-5-carboxamide 31 methyl N-[5-[6-[(4-fluorophenyl)-methyl- 1.09 464 A 212- carbamoyl]-4- 216 (methoxymethyl)benzimidazol-1-yl]-2- pyridyl]carbamate 32 methyl N-[5-[2-[(4-fluoro-3-methoxy- 1.33 466 A 220- phenyl)-methyl-carbamoyl]-6-methyl- 222 purin-9-yl]-2-pyridyl]carbamate 33 methyl N-[5-[6-(6-fluoro-3,4-dihydro-2H- 1.20 460 A 150- quinoline-1-carbonyl)-4-methyl- 153 benzimidazol-1-yl]-2-pyridyl]carbamate
Entry IUPAC name Chemical structure RT [M+H] Method MP (min) (measured) (°C) 34 methyl N-[5-[6-[ethyl-(4- 1.16 478 A 99- fluorophenyl)carbamoyl]-4- 100 (methoxymethyl)benzimidazol-1-yl]-2- pyridyl]carbamate 35 methyl N-[5-[5-(6-fluoro-3,4-dihydro-2H- 1.21 461 A 215- quinoline-1-carbonyl)-7-methyl- 218 imidazo[4,5-b]pyridin-3-yl]-2- pyridyl]carbamate 36 methyl N-[5-[5-[(4-fluoro-3-methyl- 1.18 449 A 202- phenyl)-methyl-carbamoyl]-7-methyl- 204 imidazo[4,5-b]pyridin-3-yl]-2- pyridyl]carbamate 37 methyl N-[5-[6-[(4-fluoro-3-methoxy- 1.37 451 A 228- phenyl)-methyl-carbamoyl]imidazo[4,5- 229 b]pyridin-1-yl]-2-pyridyl]carbamate 38 methyl N-[5-[6-[(4-fluoro-3-methoxy- 0.82 450 C phenyl)-methyl- carbamoyl]benzimidazol-1-yl]-2- pyridyl]carbamate 39 methyl N-[5-[6-[(4-fluorophenyl)-methyl- 1.00 435 A 201- carbamoyl]-4-methyl-imidazo[4,5- 204 c]pyridin-1-yl]-2-pyridyl]carbamate 40 methyl N-[5-[5-[(4-fluoro-3-methyl- 1.14 435 A 174- phenyl)-methyl-carbamoyl]imidazo[4,5- 176 b]pyridin-3-yl]-2-pyridyl]carbamate 41 methyl N-[5-[6-[(4-fluorophenyl)-methyl- 1.29 421 A 265- carbamoyl]imidazo[4,5-b]pyridin-1-yl]-2- 266 pyridyl]carbamate
Entry IUPAC name Chemical structure RT [M+H] Method MP (min) (measured) (°C) 42 methyl N-[5-[6-[(4-fluorophenyl)-methyl- 3.16 421 D 205- carbamoyl]imidazo[4,5-c]pyridin-1-yl]-2- 210 pyridyl]carbamate 43 methyl N-[5-[2-[(4-fluorophenyl)-methyl- 1.00 422 A 199- carbamoyl]purin-9-yl]-2- 201 pyridyl]carbamate 44 3-[6-(cyclopropanecarbonylamino)-3- 1.16 444 A 105- pyridyl]-N-(4-fluorophenyl)-N,7- 107 dimethyl-benzimidazole-5-carboxamide 45 N-(4-fluorophenyl)-N,7-dimethyl-3-[6- 1.98 433 A 140- (methylcarbamoylamino)-3- 142 pyridyl]benzimidazole-5-carboxamide 46 N-(4-fluorophenyl)-N,7-dimethyl-3-[6- 1.10 470 A 145- (1H-pyrazole-5-carbonylamino)-3- 147 pyridyl]benzimidazole-5-carboxamide 47 N-(4-fluoro-3-methoxy-phenyl)-N,7- 1.10 500 A 247- dimethyl-3-[6-(1H-pyrazole-5- 249 carbonylamino)-3- pyridyl]benzimidazole-5-carboxamide 48 N-(4-fluoro-3-methoxy-phenyl)-N,7- 1.01 500 A 175- dimethyl-3-[6-(1H-pyrazole-4- 177 carbonylamino)-3- pyridyl]benzimidazole-5-carboxamide 49 N-(4-fluorophenyl)-N,7-dimethyl-3-[6- 1.03 500 A 267- (1H-pyrazole-4-carbonylamino)-3- 269 pyridyl]benzimidazole-5-carboxamide
Entry IUPAC name Chemical structure RT [M+H] Method MP (min) (measured) (°C) 50 N-(4-fluorophenyl)-N,7-dimethyl-3-[6- 1.08 484 A 205- [(1-methylpyrazole-4-carbonyl)amino]- 208 3-pyridyl]benzimidazole-5-carboxamide 51 N-(4-fluoro-3-methoxy-phenyl)-N,7- 1.07 514 A 204- dimethyl-3-[6-[(1-methylpyrazole-4- 207 carbonyl)amino]-3- pyridyl]benzimidazole-5-carboxamide 52 N-(4-fluoro-3-methoxy-phenyl)-N,7- 1.17 514 A 180- dimethyl-3-[6-[(2-methylpyrazole-3- 181 carbonyl)amino]-3- pyridyl]benzimidazole-5-carboxamide 53 N-(4-fluorophenyl)-N,7-dimethyl-3-[6- 1.16 484 A 204- [(2-methylpyrazole-3-carbonyl)amino]- 205 3-pyridyl]benzimidazole-5-carboxamide 54 ethyl N-[5-[6-[(4-fluorophenyl)-methyl- 1.19 448 A 143- carbamoyl]-4-methyl-benzimidazol-1- 146 yl]-2-pyridyl]carbamate 55 methyl N-[5-[4-ethyl-6-[(4-fluorophenyl)- 1.20 447 A 110- methyl-carbamoyl]benzimidazol-1-yl]-2- 112 pyridyl]carbamate 56 methyl N-[5-[2-[(4-fluorophenyl)-methyl- 1.31 436 A 189- carbamoyl]-6-methyl-purin-9-yl]-2- 191 pyridyl]carbamate 57 methyl N-[5-[5-[(4-fluorophenyl)-methyl- 2.01 422 A 258- carbamoyl]imidazo[4,5-b]pyrazin-3-yl]- 260 2-pyridyl]carbamate
Entry IUPAC name Chemical structure RT [M+H] Method MP (min) (measured) (°C) 58 methyl N-[5-[2-[(4-fluoro-3-methyl- 1.06 436 A 167- phenyl)-methyl-carbamoyl]purin-9-yl]-2- 169 pyridyl]carbamate 59 methyl N-[5-[2-(6-fluoro-3,4-dihydro-2H- 1.08 448 A 226- quinoline-1-carbonyl)purin-9-yl]-2- 228 pyridyl]carbamate 60 methyl N-[5-[5-(6-fluoro-3,4-dihydro-2H- 1.10 447 A 195- quinoline-1-carbonyl)imidazo[4,5- 198 b]pyridin-3-yl]-2-pyridyl]carbamate 61 methyl N-[5-[6-(6-fluoro-3,4-dihydro-2H- 1.15 447 A 222- quinoline-1-carbonyl)imidazo[4,5- 225 b]pyridin-1-yl]-2-pyridyl]carbamate 62 methyl N-[5-[5-[(4-fluorophenyl)-methyl- 1.07 421 A 198- carbamoyl]imidazo[4,5-b]pyridin-3-yl]-2- 203 pyridyl]carbamate 63 methyl N-[5-[6-[(4-fluoro-3-methyl- 1.36 435 A 211- phenyl)-methyl-carbamoyl]imidazo[4,5- 212 b]pyridin-1-yl]-2-pyridyl]carbamate 64 methyl N-[5-[6-(7-fluoro-2,3-dihydro- 1.18 463 A 225- 1,4-benzoxazine-4-carbonyl)-4-methyl- 227 benzimidazol-1-yl]-2-pyridyl]carbamate 65 methyl N-[5-[2-[(4-fluoro-3-methoxy- 1.01 452 A 165- phenyl)-methyl-carbamoyl]purin-9-yl]-2- 168 pyridyl]carbamate
Entry IUPAC name Chemical structure RT [M+H] Method MP (min) (measured) (°C) 66 methyl N-[4-[2-[(4-chlorophenyl)- 0.86 437 C 235- methyl-carbamoyl]purin-9- 238 yl]phenyl]carbamate 67 9-(4-acetamidophenyl)-N-(4- 0.79 421 C 163- chlorophenyl)-N-methyl-purine-2- 173 carboxamide 68 3-(4-acetamidophenyl)-N-(4- 0.91 420 C chlorophenyl)-N-methyl-benzotriazole- 5-carboxamide 69 methyl N-[4-[6-[(4-chlorophenyl)- 0.97 436 C methyl-carbamoyl]benzotriazol-1- yl]phenyl]carbamate 70 1-(4-acetamidophenyl)-N-(4- 11.5 420 D 110- chlorophenyl)-N-methyl-imidazo[4,5- 120 c]pyridine-6-carboxamide 71 N-(4-chlorophenyl)-3-[6-[(2- 1.03 464 B 103- methoxyacetyl)amino]-3-pyridyl]-N,7- 105 dimethyl-benzimidazole-5-carboxamide 72 N-(4-fluoro-3-methoxy-phenyl)-3-[6-[(2- 1.16 478 B 110- methoxyacetyl)amino]-3-pyridyl]-N,7- 112 dimethyl-benzimidazole-5-carboxamide 73 N-(3,4-difluorophenyl)-3-[6-[(2- 1.06 466 B 98- methoxyacetyl)amino]-3-pyridyl]-N,7- 100 dimethyl-benzimidazole-5-carboxamide
Entry IUPAC name Chemical structure RT [M+H] Method MP (min) (measured) (°C) 74 3-[4- 1.04 461 B 120- (cyclopropanecarbonylamino)phenyl]- 122 N-(3,4-difluorophenyl)-N,7-dimethyl- benzimidazole-5-carboxamide 75 3-(6-acetamido-3-pyridyl)-N-(4-fluoro-3- 1.00 432 B 96-98 methyl-phenyl)-N,7-dimethyl- benzimidazole-5-carboxamide 76 3-[6-(cyclopropanecarbonylamino)-3- 1.06 458 B 142- pyridyl]-N-(4-fluoro-3-methyl-phenyl)- 144 N,7-dimethyl-benzimidazole-5- carboxamide 77 tert-butyl N-[4-[6-[(3,4-difluorophenyl)- 0.99 479 B 112- methyl-carbamoyl]benzimidazol-1- 114 yl]phenyl]carbamate 78 3-[6-(cyclopropanecarbonylamino)-3- 1.00 460 B 84-86 pyridyl]-N-(4-fluoro-3-methoxy-phenyl)- N-methyl-benzimidazole-5-carboxamide 79 methyl N-[4-[6-[(4-fluoro-3-methoxy- 1.04 463 B 175- phenyl)-methyl-carbamoyl]-4-mCSethyl- 177 benzimidazol-1-yl]phenyl]carbamate 80 tert-butyl N-[4-[6-[(3,4-difluorophenyl)- 1.19 493 B 189- methyl-carbamoyl]-4-methyl- 191 benzimidazol-1-yl]phenyl]carbamate 81 benzyl N-[4-[6-[(3,4-difluorophenyl)- 1.20 527 B 84-86 methyl-carbamoyl]-4-methyl- benzimidazol-1-yl]phenyl]carbamate
Entry IUPAC name Chemical structure RT [M+H] Method MP (min) (measured) (°C) 82 methyl N-[4-[6-[(3,4-difluorophenyl)- 1.05 451 B 105- methyl-carbamoyl]-4-methyl- 107 benzimidazol-1-yl]phenyl]carbamate 83 3-(4-acetamidophenyl)-N-(3,4- 1.00 435 B 128- difluorophenyl)-N,7-dimethyl- 130 benzimidazole-5-carboxamide 84 benzyl N-[4-[6-[(4-fluoro-3-methoxy- 1.18 539 B 83-85 phenyl)-methyl-carbamoyl]-4-methyl- benzimidazol-1-yl]phenyl]carbamate 85 tert-butyl N-[4-[6-[(4-fluoro-3-methoxy- 1.18 505 B 150- phenyl)-methyl-carbamoyl]-4-methyl- 152 benzimidazol-1-yl]phenyl]carbamate 86 N-(4-cyanophenyl)-3-[6- 1.01 451 B 144- (cyclopropanecarbonylamino)-3- 146 pyridyl]-N,7-dimethyl-benzimidazole-5- carboxamide 87 N-(4-chlorophenyl)-3-[6- 1.05 460 B 155- (cyclopropanecarbonylamino)-3- 157 pyridyl]-N,7-dimethyl-benzimidazole-5- carboxamide 88 N-(4-fluoro-3-methoxy-phenyl)-3-(6- 0.91 420 B 214- formamido-3-pyridyl)-N-methyl- 216 benzimidazole-5-carboxamide 89 N-(4-cyano-3-methoxy-phenyl)-3-[6- 1.02 481 B 150- (cyclopropanecarbonylamino)-3- 152 pyridyl]-N,7-dimethyl-benzimidazole-5- carboxamide
Entry IUPAC name Chemical structure RT [M+H] Method MP (min) (measured) (°C) 90 N-(4-fluoro-3-methoxy-phenyl)-3-[6-[(2- 0.98 464 B methoxyacetyl)amino]-3-pyridyl]-N- methyl-benzimidazole-5-carboxamide 91 methyl N-[5-[6-[(4-cyanophenyl)-methyl- 0.99 441 B 200- carbamoyl]-4-methyl-benzimidazol-1- 202 yl]-2-pyridyl]carbamate 92 3-(6-acetamido-3-pyridyl)-N-(3,4- 0.99 436 B 67-69 difluorophenyl)-N,7-dimethyl- benzimidazole-5-carboxamide 93 3-(4-acetamidophenyl)-N-(4-fluoro-3- 0.94 434 B methoxy-phenyl)-N-methyl- benzimidazole-5-carboxamide 94 3-(6-acetamido-3-pyridyl)-N-(4-fluoro-3- 0.97 478 B methoxy-phenyl)-N-(methoxymethyl)-7- methyl-benzimidazole-5-carboxamide 95 methyl N-[5-[6-[(4-chlorophenyl)- 1.04 450 B 208- methyl-carbamoyl]-4-methyl- 210 benzimidazol-1-yl]-2-pyridyl]carbamate 96 methyl N-[5-[6-[(3,4-difluorophenyl)- 1.02 451 B 201- methyl-carbamoyl]-4-methyl- 203 benzimidazol-1-yl]-2-pyridyl]carbamate 97 3-[6-(cyclopropanecarbonylamino)-3- 1.08 504 B pyridyl]-N-(4-fluoro-3-methoxy-phenyl)- N-(methoxymethyl)-7-methyl- benzimidazole-5-carboxamide
Entry IUPAC name Chemical structure RT [M+H] Method MP (min) (measured) (°C) 98 methyl N-[5-[6-[(4-chlorophenyl)- 1.14 466 B 217- methyl-carbamothioyl]-4-methyl- 219 benzimidazol-1-yl]-2-pyridyl]carbamate Example 1: Preparation of methyl N-[4-[6-[(4-chlorophenyl)-methyl-carbamoyl]benzotriazol-1- yl]phenyl]carbamate (compound 69)

To a mixture of 4-[tert-butoxycarbonyl(methyl)amino]benzoic acid (CAS 263021-30-3, 600 mg, 1.91 mmol) and 4-chloro-N-methylaniline (307 mg, 0.262 mL, 2.10 mmol, 1.10 eq.) in dichloromethane (36.0 mL) were added N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (411 mg, 2.10 mmol, 1.10 eq.), 1-hydroxy-7-azabenzotriazole (298 mg, 2.10 mmol, 1.10 eq.) and pyridine (0.468 mL, 5.73 mmol, 3.00 eq.). The reaction mixture was stirred at room temperature overnight, then the yellow solution was diluted with dichloromethane and quenched with water. The organic phase was separated, washed successively with water and brine, dried over magnesium sulfate, filtered, and concentrated under reduced pressure. The crude residue was purified by flash chromatography over silica gel (cyclohexane/ethyl acetate) to afford tert-butyl N-[4-[(4-chlorophenyl)-methyl-carbamoyl]phenyl]-N- methyl-carbamate as light yellow crystals. LC/MS (Method C) retention time = 1.12 min; [M+H]
+ = 375
1H NMR (400 MHz, CDCl3, ppm) δ = 7.24 - 7.28 (m, 2H), 7.18 - 7.23 (m, 2H), 7.07 – 7.11 (m, 2H), 6.96 - 7.01 (m, 2H), 3.47 (s, 3H), 3.21 (s, 3H), 1.41 (s, 9H). Step 2: Preparation of N-(4-chlorophenyl)-N-methyl-4-(methylamino)benzamide
To a solution of tert-butyl N-[4-[(4-chlorophenyl)-methyl-carbamoyl]phenyl]-N-methyl-carbamate (515 mg, 1.24 mmol) in dichloromethane (21.0 mL) under argon at 0 °C was added trifluoroacetic acid (1.44 mL, 18.5 mmol, 15.0 eq.) dropwise. The reaction mixture was then warmed to room temperature and stirred for an additional 5 hours. The mixture was then quenched with saturated NaHCO3 until pH 9 and extracted with dichloromethane. The organic layer was washed with water and brine, dried over sodium sulfate, filtered, and concentrated under reduced pressure to afford N-(4-chlorophenyl)-N-methyl-4- (methylamino)benzamide. The crude product was used without further purification. LC/MS (Method C) retention time = 0.95 min; [M+H]
+ = 275
1H NMR (400 MHz, CDCl3, ppm) δ = 7.17 - 7.25 (m, 4H), 6.96 - 7.06 (m, 2H), 6.35 - 6.39 (m, 2H), 3.46 (s, 3H), 2.82 (s, 3H), 3.97 (br s, 1H). Step 3: Preparation of tert-butyl N-[3-bromo-4-[(E)-[4-[(4-chlorophenyl)-methyl-carbamoyl]-N-methyl- anilino]azo]phenyl]carbamate

To a solution of tert-butyl N-(4-amino-3-bromo-phenyl)carbamate (CAS 1554844-65-3, prepared as described in WO2014140075A1) (260 mg, 0.860 mmol) in acetonitrile (2.60 mL) at 0 °C was added aqueous HCl 32% (0.422 mL, 4.30 mmol, 5.00 eq.). This mixture was cooled to -10 °C and stirred for 15 minutes, after which a solution of sodium nitrite (63.6 mg, 0.903 mmol, 1.05 eq.) in cold water (1.00 mL) was added dropwise. The resulting diazonium salt was stirred for 30 minutes at -10 °C and then added to a mixture of N-(4-chlorophenyl)-N-methyl-4-(methylamino)benzamide (283 mg, 0.946 mmol, 1.10 eq.) and potassium carbonate (300 mg, 2.15 mmol, 2.50 eq.) in acetonitrile/water (1:2, 7.80 mL) at -10 °C. The reaction mixture was stirred at -10 °C for an additional 2 hours and then slowly warmed to room temperature, then it was diluted with ethyl acetate and quenched with water. The aqueous layer was extracted with ethyl acetate and the combined organic layers were washed with brine, dried over sodium sulfate, filtered, and concentrated under reduced pressure. The crude residue was purified flash chromatography over silica gel (cyclohexane/ethyl acetate) to afford tert-butyl N-[3-bromo-4-[(E)-[4-[(4- chlorophenyl)-methyl-carbamoyl]-N-methyl-anilino]azo]phenyl]carbamate as yellow crystals. LC/MS (Method C) retention time = 1.31 min; [M+H]
+ = 574
1H NMR (400 MHz, CDCl3, ppm) δ = 7.86 (s, 1H), 7.49 (d, J = 8.8 Hz, 1H), 7.35 - 7.39 (m, 2H), 7.29 - 7.34 (m, 2H), 7.24 - 7.27 (m, 2H), 7.19 - 7.23 (m, 1H), 7.00 - 7.07 (m, 2H), 6.51 (s, 1H), 3.65 (s, 3H), 3.51 (s, 3H), 1.53 - 1.56 (m, 9H). Step 4: Preparation of tert-butyl N-[4-[6-[(4-chlorophenyl)-methyl-carbamoyl]benzotriazol-1- yl]phenyl]carbamate
To a solution of tert-butyl N-[3-bromo-4-[(E)-[4-[(4-chlorophenyl)-methyl-carbamoyl]-N-methyl- anilino]azo]phenyl]carbamate (150 mg, 0.249 mmol) in diemthylformamide (3.00 mL) was added potassium acetate (29.6 mg, 0.298 mmol, 1.20 eq.) and the mixture was purged with argon for 5 minutes. Palladium(II) acetate (2.9 mg, 0.012 mmol, 0.050 eq.) and 1,3-bis(diphenylphosphino)propane (15.7 mg, 0.0373 mmol, 0.150 eq.) were added to the yellow solution, the vial was sealed and stirred overnight at 100°C. The mixture was diluted with tert-butyl methyl ether, quenched with water and extracted with tert-butyl methyl ether. The organic layers were washed with water and brine, dried over sodium sulfate, filtered, and concentrated under reduced pressure. The crude residue was purified by flash chromatography over silica gel (tert-butyl methyl ether /ethyl acetate) to afford tert-butyl N-[4-[6-[(4- chlorophenyl)-methyl-carbamoyl]benzotriazol-1-yl]phenyl]carbamate as a brown resin. LC/MS (Method C) retention time = 1.13 min; [M+H]
+ = 478
1H NMR (400 MHz, CDCl3, ppm) δ = 7.96 (dd, J = 8.8, 0.7 Hz, 1H), 7.66 (s, 1H), 7.59 - 7.64 (m, 2H), 7.45 - 7.51 (m, 2H), 7.39 (dd, J = 8.6, 1.3 Hz, 1H), 7.18 - 7.27 (m, 2H), 6.97 - 7.07 (m, 2H), 6.73 (s, 1H), 3.24 (s, 3H), 1.21 (s, 9H). Step 5: Preparation of 3-(4-aminophenyl)-N-(4-chlorophenyl)-N-methyl-benzotriazole-5-carboxamide

To a solution of tert-butyl N-[4-[6-[(4-chlorophenyl)-methyl-carbamoyl]benzotriazol-1- yl]phenyl]carbamate (84.0 mg, 0.162 mmol) in dichloromethane (4.00 mL) at 0 °C under argon was added trifluoroacetic acid (0.189 mL, 2.43 mmol, 15.0 eq.). The resulting mixture was then slowly warmed to room temperature and stirred overnight. The mixture was then evaporated, the resulting resin was diluted with dichloromethane, treated with sat. NaHCO3 and extracted with dichloromethane. The combine organic layers were washed with water and brine, dried over sodium sulfate, filtered, and concentrated under reduced pressure. The crude residue was purified by flash chromatography over silica gel (tert-butyl methyl ether /ethyl acetate) to afford 3-(4-aminophenyl)-N-(4-chlorophenyl)-N- methyl-benzotriazole-5-carboxamide as an orange resin. LC/MS (Method C) retention time = 0.91 min; [M+H]
+ = 378
1H NMR (400 MHz, CDCl3, ppm) δ = 7.92 - 7.96 (m, 1H), 7.62 (s, 1H), 7.38 (dd, J = 8.4, 1.5 Hz, 1H), 7.28 - 7.30 (m, 2H), 7.23 - 7.27 (m, 2H), 6.99 - 7.05 (m, 2H), 6.82 - 6.87 (m, 2H), 3.98 (br s, 2H), 3.54 (s, 3H). Step 6: Preparation of methyl N-[4-[6-[(4-chlorophenyl)-methyl-carbamoyl]benzotriazol-1- yl]phenyl]carbamate (Compound 69) To a solution of 3-(4-aminophenyl)-N-(4-chlorophenyl)-N-methyl-benzotriazole-5-carboxamide (30.0 mg, 0.0731 mmol) and pyridine (0.018 mL, 0.22 mmol, 3.00 eq.) in dichloromethane (1.00 mL) at 10°C was added methyl chloroformate (0.014 mL, 0.18 mmol, 2.5 eq.) dropwise, after which the reaction mixture was allowed to warm to room temperature and stirred for an additional 2 hours. The reaction mixture was then diluted with dichloromethane, quenched with saturated aqueous NaHCO3, and the aqueous layer was extracted with dichloromethane. The organic layers were washed with brine, dried over sodium sulfate, filtered, and concentrated under reduced pressure. The crude residue was purified by flash chromatography over silica gel (tert-butyl methyl ether/ethyl acetate) to afford methyl N-[4-[6- [(4-chlorophenyl)-methyl-carbamoyl]benzotriazol-1-yl]phenyl]carbamate as yellow crystals. LC/MS (Method A) retention time = 0.97 min; [M+H]
+ = 436
1H NMR (400 MHz, CDCl3, ppm) δ = 7.97 (d, J = 8.4 Hz, 1H), 7.66 (br s, 1H), 7.64 (d, J = 8.8 Hz, 2H), 7.46 - 7.55 (m, 2H), 7.39 (dd, J = 8.8, 1.5 Hz, 1H), 7.25 - 7.28 (m, 2H), 7.03 (d, J = 8.8 Hz, 2H), 6.86 (s, 1H), 3.87 (s, 3H), 3.54 (s, 3H). Example 2: Preparation of methyl N-[4-[2-[(4-chlorophenyl)-methyl-carbamoyl]purin-9- yl]phenyl]carbamate (compound 66)
(Compound 66) Step 1: Preparation of tert-butyl N-[4-[(5-amino-2-chloro-pyrimidin-4-yl)amino]phenyl]carbamate
To a solution of 5-amino-2,4-dichloropyrimidine (2.00 g, 11.8 mmol) in toluene (80.0 mL) were added t- butyl (4-aminophenyl)carbamate (5.03 g, 23.7 mmol, 2.00 eq.) and triethylamine (5.00 mL, 35.5 mmol, 3.00 eq.). The resulting mixture heated to 110°C and stirred for 114 hours. After the reaction mixture had cooled down to room temperature,it was directly taken up on isolute and purified by flash chromatography over silica gel (ethyl acetate/methanol) to afford tert-butyl N-[4-[(5-amino-2-chloro- pyrimidin-4-yl)amino]phenyl]carbamate as a purple solid.
LC/MS (Method C) retention time = 0.91 min; [M+H]
+ = 336
1H NMR (400 MHz, CDCl3, ppm) δ = 7.79 (s, 1H), 7.51 - 7.54 (m, 2H), 7.36 (br d, J = 8.8 Hz, 2H), 7.02 (s, 1H), 6.49 (br s, 1H), 3.11 (br s, 2H), 1.53 (m, 9H). Step 2: Preparation of tert-butyl N-[4-(2-chloropurin-9-yl)phenyl]carbamate

To a solution of tert-butyl N-[4-[(5-amino-2-chloro-pyrimidin-4-yl)amino]phenyl]carbamate (2.60 g, 6.97 mmol) in acetic acid (15.6 mL) was added triethyl orthoformate (17.6 mL, 105 mmol, 15.0 eq.) and the reaction mixture was stirred at 120°C overnight. The reaction mixture was then cooled and the acetic acid was removed under reduced pressure. The resulting residue was diluted with water, basified with aqueous Na2CO3, and extracted with ethyl acetate. The combined organic layers were washed with water and brine, dried over sodium sulfate, filtered, and concentrated under reduced pressure. The crude product was purified by flash chromatography (ethyl acetate/methanol) to afford the desired compound, which was further purified by crystallization using a mixture of chloroform and ethyl acetate. The solid was filtered and dried under reduced pressure to afford tert-butyl N-[4-(2-chloropurin-9- yl)phenyl]carbamate as an off-white solid. LC/MS (Method C) retention time = 0.97 min; [M+H]
+ = 346
1H NMR (400 MHz, CDCl3, ppm) δ = 9.10 (s, 1H), 8.41 (s, 1H), 7.61 (app s, 4H), 6.74 (br s, 1H), 1.55 (s, 9H). Step 3: Preparation of methyl 9-[4-(tert-butoxycarbonylamino)phenyl]purine-2-carboxylate
To a solution of 1,1'-bis(diphenylphosphino)ferrocene (35 mg, 0.061 mmol, 0.04 eq.) and bis(benzonitrile) palladium(II) chloride (12 mg, 0.031 mmol, 0.02 eq.) in methanol (22.0 mL) was added tert-butyl N-[4-(2-chloropurin-9-yl)phenyl]carbamate (567 mg, 1.52 mmol) and triethylamine (0.279 mL, 1.98 mmol, 1.30 eq.). The reaction mixture was purged with argon for 5 minutes, then it was stirred overnight at 80 °C under 10 bar of CO. After cooling and purging the CO, the mixture was directly taken up on isolute and purified by flash chromatography over silica gel (ethyl acetate/methanol) to afford methyl 9-[4-(tert-butoxycarbonylamino)phenyl]purine-2-carboxylate as a light red solid. LC/MS (Method C) retention time = 0.87 min; [M+H]- = 368
1H NMR (400 MHz, CDCl3, ppm) δ = 9.39 (s, 1H), 8.50 (s, 1H), 7.59 - 7.69 (m, 4H), 6.91 (s, 1H), 4.09 (s, 3H), 1.52 (s, 9H).
Step 4: Preparation of 9-[4-(tert-butoxycarbonylamino)phenyl]purine-2-carboxylic acid

To a solution of 9-[4-(tert-butoxycarbonylamino)phenyl]purine-2-carboxylate (560 mg, 1.39 mmol) in tetrahydrofuran/water (3:1, 14.0 mL) at 5 °C was added lithium hydroxide (89.6 mg, 2.09 mmol, 1.50 eq.). The reaction mixture was allowed to warm room temperature and then stirred for an additional 5 hours, after which it was diluted with water and the tetrahydrofuran was evaporated under reduced pressure. The resulting aqueous mixture was extracted with tert-butyl methyl ether, the aqueous layer was then acidified with 1N HCl to pH 3 and extracted with ethyl acetate. The combined ethyl acetate layers were washed with brine, dried over sodium sulfate, filtered, and concentrated under reduced pressure to afford the crude 9-[4-(tert-butoxycarbonylamino)phenyl]purine-2-carboxylic acid as beige crystals. The product was used in the next step without further purification. LC/MS (Method C) retention time = 0.78 min; [M+H]
+ = 356
1H NMR (400 MHz, DMSO-d6) δ ppm 13.29 (br s, 1H), 9.69 (s, 1H), 9.37 (s, 1H), 9.11 (s, 1H), 7.78 (d, J = 9.2 Hz, 2H), 7.71 (d, J = 9.2 Hz, 2H), 1.51 (m, 9H). Step 5: Preparation of tert-butyl N-[4-[2-[(4-chlorophenyl)-methyl-carbamoyl]purin-9- yl]phenyl]carbamate

A mixture of 9-[4-(tert-butoxycarbonylamino)phenyl]purine-2-carboxylic acid (490 mg, 1.27 mmol), 4- chloro-N-methylaniline (0.174 mL, 1.40 mmol, 1.10 eq.), N-(3-dimethylaminopropyl)-N′- ethylcarbodiimide hydrochloride (273 mg, 1.40 mmol, 1.10 eq.), 1-hydroxy-7-azabenzotriazole (198 mg, 1.40 mmol, 1.10 eq.) and pyridine (0.311 mL, 3.81 mmol, 3.00 eq.) was dissolved in dichloromethane (24.0 mL) under argon and stirred at room temperature overnight. The reaction mixture was diluted with additional dichloromethane, quenched with water. The organic layer was separated and washed successively with water and brine, dried over magnesium sulfate, filtered, and concentrated under reduced pressure. The crude residue was purified by flash chromatography over silica gel (ethyl acetate/methanol) to afford tert-butyl N-[4-[2-[(4-chlorophenyl)-methyl-carbamoyl]purin-9- yl]phenyl]carbamate as colorless gum.
LC/MS (Method C) retention time = 1.02 min; [M+H]
+ = 479
1H NMR (400 MHz, CDCl3, ppm) δ = 9.11 (br s, 1H), 8.31 (s, 1H), 7.56 (br d, J = 8.8 Hz, 2H), 7.31 (br d, J = 7.7 Hz, 2H), 7.16 (br d, J = 8.1 Hz, 2H), 7.05 (br d, J = 7.3 Hz, 2H), 6.80 (br s, 1H), 3.53 (br s, 3H), 1.55 (s, 9H). Step 6: Preparation of 9-(4-aminophenyl)-N-(4-chlorophenyl)-N-methyl-purine-2-carboxamide

To a solution of tert-butyl N-[4-[2-[(4-chlorophenyl)-methyl-carbamoyl]purin-9-yl]phenyl]carbamate (410 mg, 0.788 mmol) in methanol (10.0 mL) at 0 °C was added HCl (4 M in dioxane, 2.00 mL, 7.88 mmol, 10.0 eq.) dropwise. The reaction mixture was stirred at room temperature for 72 hours, then it was concentrated under reduced pressure, the residue was stirred in a mixture of water and ethyl acetate and basic layer was basified with NaOH to pH 9-10. The mixture was extracted with ethyl acetate and the combined organic layers were washed with water and brine, dried over sodium sulfate, filtered, and concentrated under reduced pressure. The crude residue was purified by flash chromatography over silica gel (ethyl acetate/methanol) to afford 9-(4-aminophenyl)-N-(4-chlorophenyl)-N-methyl-purine-2- carboxamide as a beige solid. LC/MS (Method A) retention time = 0.78 min; [M+H]
+ = 379
1H NMR (400 MHz, CDCl3, ppm) δ = 9.11 (s, 1H), 8.26 (s, 1H), 7.01 - 7.25 (m, 6H), 6.79 (d, J = 8.4 Hz, 2H), 3.96 (br s, 2H), 3.54 (br s, 3H). Step 7: Preparation of methyl N-[4-[2-[(4-chlorophenyl)-methyl-carbamoyl]purin-9-yl]phenyl]carbamate (Compound 66) To a solution of 9-(4-aminophenyl)-N-(4-chlorophenyl)-N-methyl-purine-2-carboxamide (66.0 mg, 0.166 mmol) and pyridine (0.041 mL, 0.50 mmol, 3.0 eq.) in dichloromethane (3.00 mL) at 10 °C was added methyl chloroformate (0. mL, 0.41 mmol, 2.5 eq.) dropwise. The reaction mixture was then allowed to warm to room temperature and stirred for an additional 2 hours. The reaction mixture was then diluted with dichloromethane, quenched with aqueous NaHCO3, and extracted with dichloromethane. The combined organic layers were washed with brine, dried over sodium sulfate, filtered, and concentrated under reduced pressure. The crude residue was purified by flash chromatography over silica gel (cyclohexane/ethyl acetate) to afford methyl N-[4-[2-[(4-chlorophenyl)-methyl-carbamoyl]purin-9- yl]phenyl]carbamate as a white solid. LC/MS (Method C) retention time = 0.86 min; [M+H]
+ = 437
1H NMR (400 MHz, CDCl3, ppm) δ = 9.10 (s, 1H), 8.32 (s, 1H), 7.59 (br d, J = 8.4 Hz, 2H), 7.32 (br d, J = 8.1 Hz, 2H), 7.15 (br s, 5H), 7.06 (br d, J = 7.7 Hz, 2H), 3.80 (s, 3H), 3.54 (s, 3H).
Example 3: Preparation of methyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]-4- methyl-benzimidazol-1-yl]-2-pyridyl]carbamate (compound 26)
(Compound 26) Step 1: Preparation of 3-tert-butoxycarbonyl-7-methyl-benzimidazole-5-carboxylic acid
To a yellow suspension of 7-methyl-3H-benzimidazole-5-carboxylic acid (CAS: 398452-96-5; 7.0 g, 40 mmol) in acetonitrile (160 mL) and water (1.50 mL) was added triethylamine (11.0 mL, 79.0 mmol, 2.00 eq.) followed by 4-dimethylaminopyridine (490 mg, 4.00 mmol, 0.10 eq.) and a portion wise addition of di-tert-butyl dicarbonate (11.0 mL, 48.0 mmol, 1.20 eq.). The reaction mixture was stirred at room temperature for 2 hours, then it was diluted with ethyl acetate and water. The aqueous layer was acidified with HCl 2M to pH 1 and the mixture was extracted with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate, filtered, and concentrated under reduced pressure to afford 3-tert-butoxycarbonyl-7-methyl-benzimidazole-5-carboxylic acid as a pale yellow solid. LC/MS (Method C) retention time = 0.89 min; [M+H]- = 275
1H NMR (400 MHz, DMSO-d6, ppm) δ = 12.95 (br s, 1H), 8.76 (s, 1H), 8.41 (s, 1H), 7.80 (s, 1H), 2.60 (s, 3H), 1.67 (s, 9H). Step 2: Preparation of tert-butyl 6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]-4-methyl- benzimidazole-1-carboxylate

To a solution of 4-fluoro-3-methoxy-N-methylaniline (320 mg, 1.96 mmol, 1.05 eq.), N,N- diisopropylethylamine (0.96 mL, 5.60 mmol, 3.0 eq.) and 3-tert-butoxycarbonyl-7-methyl-benzimidazole- 5-carboxylic acid (516 mg, 1.87 mmol) in ethyl acetate (7.5 mL) was added propylphosphonic anhydride solution (50 wt% in ethyl acetate, 2.0 mL, 3.36 mmol, 1.8 eq.), portionwise. The reaction mixture was allowed to stir at room temperature for 4 hours, then it was poured into water and extracted with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate, filtered, and concentrated under reduced pressure. The crude residue was purified by flash chromatography (cyclohexane/ethyl acetate) to afford tert-butyl 6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]-4- methyl-benzimidazole-1-carboxylate as a brown gum. LC/MS (Method C) retention time = 1.04 min; [M+H]
+ = 414
1H NMR (400 MHz, CDCl3, ppm) δ = 8.39 (s, 1H), 7.79 (s, 1H), 7.26 (d, J = 0.7 Hz, 1H), 6.92 (dd, J = 10.9, 8.7 Hz, 1H), 6.72 (dd, J = 7.4, 2.4 Hz, 1H), 6.61 - 6.66 (m, 1H), 3.75 (s, 3H), 3.51 (s, 3H), 2.57 (s, 3H), 1.68 (s, 9H). Step 3: Preparation of N-(4-fluoro-3-methoxy-phenyl)-N,7-dimethyl-3H-benzimidazole-5-carboxamide
A mixture of tert-butyl 6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]-4-methyl-benzimidazole-1- carboxylate (795 mg g, 1.92 mmol), toluene (5.8 mL) and trifluoroacetic acid (1.03 mL, 13.5 mmol, 7.00 eq.) was stirred overnight at room temperature, after which it was concentrated under reduced pressure and taken up in water. The mixture was neutralized with saturated aqueous NaHCO3 solution and extracted with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate, filtered, and concentrated under reduced pressure to afford the crude N-(4-fluoro-3-methoxy- phenyl)-N,7-dimethyl-3H-benzimidazole-5-carboxamide as a brown gum. The product was used in the next step without further purification. LC/MS (Method C) retention time = 0.67 min; [M+H]
+ = 314
1H NMR (400 MHz, CDCl3, ppm) δ = 8.37 (s, 1H), 7.77 (s, 1H), 7.25 (s, 1H), 6.90 (dd, J = 10.9, 8.7 Hz, 1H), 6.71 (dd, J = 7.6, 2.5 Hz, 1H), 6.58 - 6.66 (m, 1H), 3.74 (s, 3H), 3.50 (s, 3H), 2.56 (s, 3H). Step 4: Preparation of methyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]-4-methyl- benzimidazol-1-yl]-2-pyridyl]carbamate (Compound 26) To a mixture of N-(4-fluoro-3-methoxy-phenyl)-N,7-dimethyl-3H-benzimidazole-5-carboxamide (100 mg, 0.319 mmol), boric acid (60 mg, 0.958 mmol, 3.0 eq.) and copper (II) acetate (174 mg, 0.958 mmol, 3.0 eq.) in acetonitrile (1.0 mL) was added N,N-diisopropylethylamine (0.170 mL, 0.958 mmol, 3.0 eq.), followed by dropwise addition of a suspension of methyl N-[5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan- 2-yl)-2-pyridyl]carbamate (266 mg, 0.958 mmol, 3.0 eq.) in acetonitrile (2.0 mL). The reaction mixture was stirred at 60°C overnight. The reaction mixture was then cooled to room temperature and concentrated under reduced pressure. The resulting residue was dissolved in ethanol, filtered over a
pad of celite and the filtrate concentrated under reduced pressure. The crude residue was purified by flash chromatography over silica gel (cyclohexane/ethyl acetate) and then rinsed with acetonitrile to afford methyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]-4-methyl-benzimidazol-1-yl]-2- pyridyl]carbamate as an off white solid. LC/MS (Method C) retention time = 0.86 min; [M+H]
+ = 464
1H NMR (400 MHz, CDCl3, ppm) δ = 8.22 (d, J = 2.2 Hz, 1H), 8.19 (d, J = 8.7 Hz, 1H), 8.04 (s, 1H), 7.72 (br s, 1H), 7.55 (dd, J = 8.7, 2.5 Hz, 1H), 7.27 - 7.29 (m, 1H), 7.15 (br s, 1H), 6.94 (dd, J = 10.7, 8.5 Hz, 1H), 6.67 (dd, J = 7.4, 2.4 Hz, 1H), 6.60 (ddd, J = 8.6, 3.7, 2.5 Hz, 1H), 3.88 (s, 3H), 3.72 (s, 3H), 3.48 (s, 3H), 2.62 (s, 3H). Example 4: Preparation of N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]imidazo[4,5- c]pyridin-1-yl]-2-pyridyl]carbamate (compound 24)
Step 1: Preparation of methyl N-[5-[(2-chloro-5-nitro-4-pyridyl)amino]-2-pyridyl]carbamate

To a solution of 2,4-dichloro-5-nitro-pyridine (10.5 g, 51.7 mmol) and methyl N-(5-amino-2- pyridyl)carbamate (9.10 g, 51.7 mmol, 1.0 eq.) in ethanol (150 mL) was added N,N-diethylethanamine (21.2 mL, 155 mmol, 3.0 eq.) and the reaction mixture was stirred at 100 °C for 16 hours. After cooling down to room temperature it was concentrated under reduced pressure and then water was added to the residue. The mixture was allowed to stir for 1 hour at room temperature, then the precipitate was filtered, washed with water and hexane and dried under vacuum to afford methyl N-[5-[(2-chloro-5-nitro- 4-pyridyl)amino]-2-pyridyl]carbamate as a solid. 1H NMR (400 MHz, DMSO-d6, ppm) δ = 10.37 (s, 1H), 9.92 (s, 1H), 8.97 (s, 1H), 8.27 (d, J = 2.4 Hz, 1H), 7.94 (d, J = 8.8 Hz, 1H), 7.80 (dd, J = 11.6 Hz, 2.8 Hz, 1H), 6.71 (s, 1H), 3.70 (s, 3H). Step 2: Preparation of methyl N-[5-[(5-amino-2-chloro-4-pyridyl)amino]-2-pyridyl]carbamate
To a solution of methyl N-[5-[(2-chloro-5-nitro-4-pyridyl)amino]-2-pyridyl]carbamate (250 mg, 0.734 mmol) in ethanol (15.0 mL) and water (5.00 mL) was added iron (81.9 mg, 1.47 mmol, 2.00 eq.) followed by ammonia hydrochloride (392 mg, 7.34 mmol, 10.0 eq.). The reaction mixture was stirred at 100 °C for 2 hours, then it was filtered through a pad of celite and washed with an excess of ethyl acetate. The filtrate was concentrated under reduced pressure, diluted with water, and extracted with ethyl acetate. The combined organic layers were dried over sodium sulfate, filtered and concentrated under reduced pressure to afford methyl N-[5-[(5-amino-2-chloro-4-pyridyl)amino]-2-pyridyl]carbamate as a solid. The product was used in the next step without further purification. 1H NMR (400 MHz, DMSO-d6, ppm) δ = 10.12 (s, 1H), 8.13 (d, J = 2.0 Hz, 1H), 7.83 (d, J = 8.8 Hz, 1H), 7.74 (s, 1H), 7.64 (dd, J = 11.6 Hz, 1H), 7.60 (s, 1H), 6.54 (s, 1H), 5.00 (s, 2H), 3.68 (s, 3H). Step 3: Preparation of methyl N-[5-(6-chloroimidazo[4,5-c]pyridin-1-yl)-2-pyridyl]carbamate

To a solution of methyl N-[5-[(5-amino-2-chloro-4-pyridyl)amino]-2-pyridyl]carbamate (5.00 g, 16.2 mmol) in diethoxymethoxyethane (25.0 mL) was added hydrogen chloride (36 mass %, 1.64 g, 16.2 mmol, 1.00 eq.) and the reaction mixture was stirred at 100 °C for 1 hour. After cooling down to room temperature, the mixture was concentrated under reduced pressure and the residue was treated with water and stirred for 20 minutes. The precipitate was filtered, washed with water and n-hexane, and dried under reduced pressure to afford methyl N-[5-(6-chloroimidazo[4,5-c]pyridin-1-yl)-2- pyridyl]carbamate as a solid. The product was used in the next step without further purification.
1H NMR (400 MHz, DMSO-d6, ppm) δ = 10.61 (s, 1H), 8.90 (s, 1H), 8.77 (s, 1H), 8.643 (d, J = 2.4 Hz, 1H), 8.17 (dd, J = 11.6, 2.4 Hz, 1H), 8.06 (d, J = 9.2 Hz, 1H), 7.77 (s, 1H), 3.72 (s, 3H). Step 4: Preparation of methyl 1-[6-(methoxycarbonylamino)-3-pyridyl]imidazo[4,5-c]pyridine-6- carboxylate
In a steel autoclave methyl N-[5-(6-chloroimidazo[4,5-c]pyridin-1-yl)-2-pyridyl]carbamate (3.00 g, 9.38 mmol) and sodium acetate (2.31 g, 28.2 mmol, 3.00 eq.) were dissolved in methanol (100 mL), and the mixture was degassed with argon for 10 minutes. To this mixture diphenyl-[rac-(4S,4'R)-1'- diphenylphosphanylferrocen-1-yl]phosphane (260 mg, 0.469 mmol, 0.05 eq.) and palladium(II) acetate (105 mg, 0.469 mmol, 0.05 eq.) were added, CO gas was introduced (200 psi pressure) and the reaction mixture was stirred at 100 °C for 16 hours. After cooling down to room temperature it was filtered and washed with methanol. The filtrate was concentrated under reduced pressure to afford methyl 1-[6- (methoxycarbonylamino)-3-pyridyl]imidazo[4,5-c]pyridine-6-carboxylate as a solid.
1H NMR (400 MHz, DMSO-d6, ppm) δ = 10.60 (s, 1H), 9.17 (s, 1H), 8.91 (s, 1H), 8.66 (s, 1H), 8.21 (br s, 2H), 8.10 (br s, 1H), 3.89 (s, 3H), 3.73 (S, 3H). Step 5: Preparation of lithium 1-[6-(methoxycarbonylamino)-3-pyridyl]imidazo[4,5-c]pyridine-6- carboxylate
To a solution of methyl 1-[6-(methoxycarbonylamino)-3-pyridyl]imidazo[4,5-c]pyridine-6-carboxylate (800 mg, 2.20 mmol) in a mixture of tetrahydrofuran (15.0 mL), methanol (10.0 mL) and water (10.0 mL) was added lithium hydroxide hydride (369 mg, 8.80 mmol, 4.00 eq.) and the resulting reaction mixture was stirred for 16 hours at room temperature. The mixture was then concentrated under reduced pressure, the residue was stirred in water and the precipitate was filtered and washed with methanol. The solid was dried under reduced pressure to afford lithium 1-[6-(methoxycarbonylamino)-3- pyridyl]imidazo[4,5-c]pyridine-6-carboxylate as a solid.
1H NMR (400 MHz, DMSO-d6, ppm) δ = 10.59 (s, 1H), 8.92 (app br s, 1H), 8.76 (s, 1H), 8.62 (s, 1H), 8.24 - 8.00 (m, 3H), 3.73 (s, 3H). Step 6: Preparation of methyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]imidazo[4,5- c]pyridin-1-yl]-2-pyridyl]carbamate (Compound 24)
To a solution of lithium 1-[6-(methoxycarbonylamino)-3-pyridyl]imidazo[4,5-c]pyridine-6-carboxylate (250 mg, 0.744 mmol) and 4-fluoro-3-methoxy-N-methyl-aniline (173 mg, 1.12 mmol, 1.50 eq.) in N,N- dimethylformamide (10.0 mL) was added diisopropylethylamine (0.401 mL, 2.23 mmol, 3.00 eq.) and 1- [bis(dimethylamino)-methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (565 mg, 1.49 mmol, 2.00 eq.). The resulting reaction mixture was stirred at room temperature for 16 hours, it was then diluted with water, the formed precipitate was filtered and the aqueous layer was concentrated under reduced pressure. The resulting residue was purified by reverse phase chromatography over C-18 column cartridge (acetonitrile/water) to afford methyl N-[5-[6-[(4-fluoro-3- methoxy-phenyl)-methyl-carbamoyl]imidazo[4,5-c]pyridin-1-yl]-2-pyridyl]carbamate as an off-white solid.
1H NMR (400 MHz, DMSO-d6, ppm) δ = 10.61 (s, 1H), 8.80 (br s, 1H), 8.77 (s, 1H), 8.68 (s, 1H), 8.08 (app s, 2H), 7.82 (s, 1H), 7.09 (d, J = 6.4 Hz, 1H), 7.00 (br s, 1H), 6.67 (br s, 1H), 3.73 (s, 3H), 3.65 (S, 3H), 3.39 (s, 3H). Example 5: Preparation of methyl N-[5-[6-[(4-fluorophenyl)-methyl-carbamoyl]benzimidazol-1- yl]-2-pyridyl]carbamate (compound 22)
butoxycarbonylbenzimidazole-5-carboxylic acid
To a mixture of 3H-benzimidazole-5-carboxylic acid (1.00 g, 6.04 mmol) and sodium carbonate (1.54 g, 14.5 mmol) in water (15.1 mL) at room temperature was added dropwise a solution of di-tert-butyl pyrocarbonate (2.66 g, 12.1 mmol) in dioxane (9.0 mL). The reaction mixture was stirred at room temperature overnight and then poured in water. The mixture was extracted with diethylether, the aqueous layer was acidified with HCl 2M until pH 1, and the aqueous layer extracted again with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate and concentrated under reduced pressure to yield a mixture of 1-tert-butoxycarbonylbenzimidazole-5- carboxylic acid and 3-tert-butoxycarbonylbenzimidazole-5-carboxylic acid isomers which were used in the next step without further purification.
LC/MS (Method C) retention time = 0.81 & 0.84 min; [M+H]
+ = 261 Step 2: Preparation of tert-butyl 6-[(4-fluorophenyl)-methyl-carbamoyl]benzimidazole-1-carboxylate and tert-butyl 5-[(4-fluorophenyl)-methyl-carbamoyl]benzimidazole-1-carboxylate

To a mixture of 1-tert-butoxycarbonylbenzimidazole-5-carboxylic acid and 3-tert- butoxycarbonylbenzimidazole-5-carboxylic acid (580 mg, 2.20 mmol), 4-fluoro-N-methylaniline (300 mg, 2.30 mmol, 1.05 eq.) and N,N-diisopropylethylamine (1.10 mL, 6.60 mmol, 3.00 eq.) in ethyl acetate (8.80 mL) was added propylphosphonic anhydride solution (50 wt% in ethyl acetate, 2.40 mL, 4.00 mmol, 1.80 eq.) dropwise at room temperature and the reaction mixture was stirred overnight. The mixture was then poured in water and extracted with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate, and concentrated under reduced pressure to afford a mixture of tert-butyl 6-[(4-fluorophenyl)-methyl-carbamoyl]benzimidazole-1-carboxylate and tert-butyl 5- [(4-fluorophenyl)-methyl-carbamoyl]benzimidazole-1-carboxylate isomers as a brown gum. The mixture was used in the next step without further purification. LC/MS (Method C) retention time = 0.97 & 1.00 min; [M+H]
+ = 370 Step 3: Preparation of N-(4-fluorophenyl)-N-methyl-3H-benzimidazole-5-carboxamide
A mixture of tert-butyl 6-[(4-fluorophenyl)-methyl-carbamoyl]benzimidazole-1-carboxylate and tert-butyl 5-[(4-fluorophenyl)-methyl-carbamoyl]benzimidazole-1-carboxylate (830 mg, 2.20 mmol) in a mixture of trifluoroacetic acid (1.20 mL, 16.0 mmol, 7.00 eq.) and toluene (6.70 mL) was stirred at room temperature overnight. The reaction mixture was then concentrated under reduced pressure, and the resulting residue was taken up in water and neutralized with saturated aqueous NaHCO3. The aqueous layer was extracted with ethyl acetate and the combined organic layers were washed with brine, dried over sodium sulfate, and concentrated under reduced pressure to afford N-(4-fluorophenyl)-N-methyl- 3H-benzimidazole-5-carboxamide as a beige solid. LC/MS (Method C) retention time = 0.54 min; [M+H]
+ = 270
1H NMR (400 MHz, CDCl3, ppm) δ = 8.99 - 9.50 (br s, 1H), 8.04 (s, 1H), 7.70 (br s, 1H), 7.45 (br s, 1H), 7.20 - 7.27 (m, 1H), 7.05 (dd, J = 8.9, 4.9 Hz, 2H), 6.86 - 6.95 (m, 2H), 3.53 (s, 3H).
Step 4: Preparation of methyl N-[5-[6-[(4-fluorophenyl)-methyl-carbamoyl]benzimidazol-1-yl]-2- pyridyl]carbamate (Compound 22) To a solution of N-(4-fluorophenyl)-N-methyl-3H-benzimidazole-5-carboxamide (210 mg, 0.780 mmol), boric acid (97.4 mg, 1.56 mmol, 2.00 eq.) and copper(II) acetate (212.5 mg, 1.17 mmol, 1.50 eq.) in acetonitrile (20.3 mL) at room temperature was added N,N-diisopropylethylamine (0.277 mL, 1.56 mmol, 2.00 eq.) followed by methyl N-[5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2-pyridyl]carbamate (434 mg, 1.56 mmol, 2.00 eq.). The reaction mixture was stirred at 60 °C under an atmosphere of air for 2.5 hours. The reaction mixture was cooled to room temperature and poured into water. The aqueous layer was extracted with ethyl acetate, the combined organic layers were washed with brine, dried over sodium sulfate, and concentrated under reduced pressure. The crude residue was purified over a preparative HPLC column (CO2/methanol) to afford methyl N-[5-[6-[(4-fluorophenyl)-methyl- carbamoyl]benzimidazol-1-yl]-2-pyridyl]carbamate as a pale pink solid. LC/MS (Method C) retention time = 0.82 min; [M+H]
+ = 420
1H NMR (600 MHz, CDCl3, ppm) δ = 8.28 (d, J = 2.4 Hz, 1H), 8.21 (d, J = 8.9 Hz, 1H), 8.07 (s, 1H), 7.81 (s, 1 H), 7.67 (d, J = 8.5 Hz, 1H), 7.63 (dd, J = 8.8, 2.6 Hz, 1H), 7.40 - 7.47 (m, 1H), 7.31 (dd, J = 8.4, 1.4 Hz, 1H) 7.03 (dd, J = 8.88, 4.6 Hz, 2H), 6.90 - 6.98 (m, 2 H), 3.88 (s, 3 H), 3.50 (s, 3H). Example 6: Preparation of methyl N-[5-[6-(7-fluoro-2,3-dihydro-1,4-benzoxazine-4-carbonyl)-4- methyl-benzimidazol-1-yl]-2-pyridyl]carbamate (compound 64)
(Compound 64) Step 1: Preparation of (7-fluoro-2,3-dihydro-1,4-benzoxazin-4-yl)-(7-methyl-3H-benzimidazol-5- yl)methanone

To a solution of 7-methyl-3H-benzimidazole-5-carboxylic acid (CAS 398452-96-5, commercially available and can be prepared according to WO2009144554A1) (500 mg, 2.84 mmol) in N,N- dimethylformamide (7.00 mL) was added 1-methylimidazole (699 mg, 8.51 mmol, 3.00 eq.), N,N,N',N'- tetramethylchloroformamidinium hexafluorophosphate (956 mg, 3.41 mmol, 1.20 eq.) and 7-fluoro-3,4- dihydro-2H-1,4-benzoxazine (CAS 56346-41-9, 435 mg, 2.84 mmol, 1.00 eq.). The reaction mixture was
stirred at room temperature for 4 hours, then it was diluted with water and extracted with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate, filtered, and concentrated under reduced pressure. The crude product was purified by flash chromatography over silica gel (ethyl acetate/petroleum ether) to afford (7-fluoro-2,3-dihydro-1,4-benzoxazin-4-yl)-(7-methyl- 3H-benzimidazol-5-yl)methanone as a yellow solid. LC/MS (method A) retention time = 0.91 min; [M+H]
+ = 312 Step 2: Preparation of methyl N-[5-[6-(7-fluoro-2,3-dihydro-1,4-benzoxazine-4-carbonyl)-4-methyl- benzimidazol-1-yl]-2-pyridyl]carbamate (Compound 64) To a solution of (7-fluoro-2,3-dihydro-1,4-benzoxazin-4-yl)-(7-methyl-3H-benzimidazol-5-yl)methanone (165 mg, 0.530 mmol) and methyl N-[5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2- pyridyl]carbamate (295 mg, 1.06 mmol, 2.00 eq.) in methanol (3.00 mL) was added copper(I) oxide (228 mg, 1.59 mmol, 3.00 eq.). The reaction mixture was stirred at room temperature for 24 hours under oxygen atmosphere and then it was filtered through a pad of celite, and the filtrate was concentrated under reduced pressure. The crude residue was purified by flash chromatography over silica gel (dichloromethane/methanol) first and then further by preparative HPLC chromatography (CO2/ethanol) to afford methyl N-[5-[6-(7-fluoro-2,3-dihydro-1,4-benzoxazine-4-carbonyl)-4-methyl-benzimidazol-1-yl]- 2-pyridyl]carbamate as an off-white solid. LC/MS (method A) retention time = 1.18 min; [M+H]
+ = 462 1H NMR (400 MHz, DMSO-d6, ppm) δ = 10.54 (s, 1H), 8.64 (s, 1H), 8.55 (s, 1H), 8.04 (d, J = 8.4 Hz, 2H), 7.56 (s, 1H), 7.40 (s, 1H), 7.31 (s, 1H), 6.80 (dd, J = 10.0, 2.9 Hz, 1H), 6.65 (td, J = 8.7, 2.7 Hz, 1H), 4.29 - 4.27 (m, 2H), 3.88 - 3.86 (m, 2H), 3.72 (s, 3H), 2.61 (s, 3H). Example 7: This example illustrates the preparation of methyl N-[5-[6-[(4-fluoro-3-methoxy- phenyl)-methyl-carbamoyl]-4-(methoxymethyl)benzimidazol-1-yl]-2-pyridyl]carbamate (Compound 1)

(Compound 1) Step 1: Preparation of methyl 6-bromo-1-(2-trimethylsilylethoxymethyl)benzimidazole-4-carboxylate
At 0 °C under nitrogen atmosphere added sodium hydride 60% (2.82 g, 70.6 mmol, 1.20 eq.) was added to a solution of methyl 6-bromo-1H-benzimidazole-4-carboxylate [CAS 1806519-50-9] (15.0 g, 58.8 mmol) in dry DMF (400 mL). After 20 minutes of stirring at 0 °C 2-(chloromethoxy)ethyl-trimethyl-silane (11.8 g, 70.6 mmol, 1.20 eq.) was added dropwise. The resulting reaction mixture was slowly warmed up to room temperature where it was stirred for 2 hours before it was quenched with saturated NH4Cl solution and extracted with ethyl acetate. The combined organic layers were washed with water and brine, dried over sodium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography over silica gel (eluting with dichloromethane/methanol) to afford a mixture of methyl 6-bromo-1-(2-trimethylsilylethoxymethyl)benzimidazole-4-carboxylate and methyl 6- bromo-3-(2-trimethylsilylethoxymethyl)benzimidazole-4-carboxylate as a brown solid. LC/MS (Method A) retention time = 1.46 min; [M+H]
+ = 385 Step 2: Preparation of [6-bromo-1-(2-trimethylsilylethoxymethyl)benzimidazol-4-yl]methanol
To a solution of methyl 6-bromo-1-(2-trimethylsilylethoxymethyl)benzimidazole-4-carboxylate (6.60 g, 17.1 mmol) in dry methanol (200.00 mL) was added lithium chloride (7.26 g, 171 mmol, 10.0 eq.) under an atmosphere of nitrogen. Then sodium borohydride (6.31 g, 171 mmol, 10.0 eq.) was added portion wise. The resulting reaction mixture was stirred for 12 hours at 40 °C. The reaction mixture was quenched with saturated NH4Cl solution and extracted with ehyl acetate. The combined organic layers were washed with water and brine, dried over sodium sulfate, and concentrated under reduced pressure to give [6-bromo-1-(2-trimethylsilylethoxymethyl)benzimidazol-4-yl]methanol as a brown oil. LC/MS (Method A) retention time = 1.35 min; [M+H]
+ = 357 Step 3: Preparation of 2-[[6-bromo-4-(methoxymethyl)benzimidazol-1-yl]methoxy]ethyl-trimethyl-silane
At 0 °C to a solution of [6-bromo-1-(2-trimethylsilylethoxymethyl)benzimidazol-4-yl]methanol (7.50 g, 21.0 mmol) in tetrahydrofuran (100 mL) was added portion wise sodium hydride 60% (1.01 g, 25.2 mmol, 1.20eq.). The reaction mixture was stirred for 20 minutes, then iodomethane (5.96 g, 42.0 mmol, 2.0 eq.) was added. The resulting reaction mixture was stirred for 3 hours at room temperature before it was diluted with water and extracted with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate, and concentrated under reduced pressure to give 2-[[6-bromo-4- (methoxymethyl)benzimidazol-1-yl]methoxy]ethyl-trimethyl-silane as a light yellow liquid. LC/MS (Method A) retention time = 1.42 min; [M+H]
+ = 371 Step 4: Preparation of methyl 7-(methoxymethyl)-3-(2-trimethylsilylethoxymethyl)benzimidazole-5- carboxylate
A reaction bomb was charged with 2-[[6-bromo-4-(methoxymethyl)benzimidazol-1-yl]methoxy]ethyl- trimethyl-silane (14.0 g, 37.7 mmol), methanol (350.0 mL), 1.1'-Bis(diphenylphosphino)ferrocene- palladium(II)dichloride dichloromethane complex (1.53 g, 1.89 mmol, 0.05 eq.) and N,N- diethylethanamine (11.4 g, 113 mmol, 3.0eq.). The bomb was then flushed with carbon monoxide, sealed, and pressurized to 2.5 MPa with carbon monoxide. The reaction mixture was stirred at 100 °C for 36 hours before the vessel was cooled to room temperature and the pressure released. The reaction mixture was concentrated to dryness and purified by silica gel column chromatography over silica gel (petroleum ether/ethyl acetate) to obtain methyl 7-(methoxymethyl)-3-(2- trimethylsilylethoxymethyl)benzimidazole-5-carboxylate as a brown oil. LC/MS (Method A) retention time = 1.47 min; [M+H]
+ = 351 Step 5: Preparation of 7-(methoxymethyl)-3-(2-trimethylsilylethoxymethyl)benzimidazole-5-carboxylic acid (Intermediate I-1)
(Intermediate I-1) To a stirred mixture of methyl 7-(methoxymethyl)-3-(2-trimethylsilylethoxymethyl)benzimidazole-5- carboxylate (10.0 g, 28.5 mmol) in tetrahydrofuran/water (150.0 mL) was added portion wise lithium hydroxide (2.05 g, 85.6 mmol, 3.0 eq.). The resulting reaction mixture was stirred at 40 °C for 4 hours before it was diluted with water (75.00 mL). Tetrahydrofuran was evaporated off, then it was extracted
with diethyl ether. The aqueous phase was acidified to pH = 2 with 2 M HCl aqueous solution. The precipitate was collected by filtration, washed with water and concentrated under reduces pressure to afford 7-(methoxymethyl)-3-(2-trimethylsilylethoxymethyl)benzimidazole-5-carboxylic acid as a brown solid. LC/MS (Method A) retention time = 0.75 min; [M+H]
+ = 337 Step 6: Preparation of N-(4-fluoro-3-methoxy-phenyl)-7-(methoxymethyl)-N-methyl-3-(2- trimethylsilylethoxymethyl)benzimidazole-5-carboxamide

To a solution of 4-fluoro-3-methoxy-N-methyl-aniline [CAS 904321-01-3] (300 mg, 1.93 mmol) and 7- (methoxymethyl)-3-(2-trimethylsilylethoxymethyl)benzimidazole-5-carboxylic acid (Intermediate I-1, 683 mg, 2.03 mmol, 1.05 eq.) in pyridine (15 mL) was added propylphosphonic anhydride (50.0 % in ethyl acetate, 5.80 mmol, 3.00 eq.) dropwise at 70 °C under nitrogen atmosphere. The resulting reaction mixture was stirred for 3 hours before it was quenched with saturated aqueous NaHCO3 solution and extracted with ethyl acetate. The combined organic layers were washed with water and brine, dried over sodium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography over silica gel (dichloromethane/methanol) to give N-(4-fluoro-3-methoxy-phenyl)-7- (methoxymethyl)-N-methyl-3-(2-trimethylsilylethoxymethyl) benzimidazole-5-carboxamide as a light yellow solid. LC/MS (Method A) retention time = 1.43 min; [M+H]
+ = 474 Step 7: Preparation of N-(4-fluoro-3-methoxy-phenyl)-7-(methoxymethyl)-N-methyl-3H-benzimidazole- 5-carboxamide
To a solution of N-(4-fluoro-3-methoxy-phenyl)-7-(methoxymethyl)-N-methyl-3-(2- trimethylsilylethoxymethyl) benzimidazole-5-carboxamide (750 mg, 1.58 mmol) in dichloromethane (7 mL) was added 2,2,2-trifluoroacetic acid (7 mL) dropwise at room temperature. The reaction mixture
was stirred for 8 hours at room temperature. The reaction mixture was adjusted to pH 9 with saturated aqueous NaHCO3 solution, then partitioned between dichloromethane (150 mL) and aqueous NaHCO3. The separated organic layer was evaporated to dryness and purified by silica gel column chromatography over silica gel (dichloromethane/methanol) to give N-(4-fluoro-3-methoxy-phenyl)-7- (methoxymethyl)-N-methyl-3H-benzimidazole-5-carboxamide as a light yellow solid. LC/MS (Method A) retention time = 0.86 min; [M+H]
+ = 344 Step 8: Preparation of methyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]-4- (methoxymethyl)benzimidazol-1-yl]-2-pyridyl]carbamate (Compound 1) To a solution of N-(4-fluoro-3-methoxy-phenyl)-7-(methoxymethyl)-N-methyl-3H-benzimidazole-5- carboxamide (200 mg, 0.582 mmol) and methyl N-[5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2- pyridyl]carbamate [CAS 1073372-02-7] (324 mg, 1.16 mmol, 2.0eq.) in methanol (15.0 mL) was added copper(I) oxide (250 mg, 1.75 mmol, 3.0 eq.). The reaction mixture was stirred at 35 °C for 24 hours under oxygen atmosphere. Then the mixture was filtered through celite. The filtrate was concentrated under reduces pressure to give the crude which was purified by column chromatography on silica gel (methanol/dichloromethane). It was further purified by SFC and then trituration in diethyl ether to afford methyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]-4-(methoxymethyl)benzimidazol-1-yl]- 2-pyridyl]carbamate as an off-white powder LC/MS (Method A) retention time = 1.10 min; [M+H]
+ = 494
1H NMR (400 MHz, DMSO-d6, ppm) δ = 10.56 (s, 1H), 8.56 (s, 1H), 8.47 - 8.42 (m, 1H), 8.05 (dd, J = 8.9, 0.7 Hz, 1H), 7.84 (dd, J = 8.9, 2.8 Hz, 1H), 7.40 (d, J = 1.5 Hz, 1H), 7.31 (d, J = 1.5 Hz, 1H), 7.22 (dd, J = 7.9, 2.5 Hz, 1H), 7.01 (dd, J = 11.3, 8.6 Hz, 1H), 6.65 (ddd, J = 8.6, 3.9, 2.5 Hz, 1H), 4.75 (s, 2H), 3.74 (s, 3H), 3.69 (s, 3H), 3.37 (s, 3H), 3.21 (s, 3H) Example 8: Preparation of methyl N-[5-[6-[2-cyanoethyl-(4-fluoro-3-methoxy-phenyl)carbamoyl]- 4-methyl-benzimidazol-1-yl]-2-pyridyl]carbamate (Compound 2)

(Compound 2) Step 1: Preparation of 7-methyl-3-tetrahydropyran-2-yl-benzimidazole-5-carboxylic acid (Intermediate I- 2)
(Intermediate I-2) To a suspension of 7-methyl-3H-benzimidazole-5-carboxylic acid [CAS 398452-96-5] (3.00 g, 17.0 mmol) in tetrahydrofuran (250.0 mL) was added 3,4-dihydro-2H-pyran [CAS 110-87-2] (28.6 g, 341 mmol, 20 eq.) and (7,7-dimethyl-2-oxo-norbornan-1-yl)methanesulfonic acid (0.396 g, 1.70 mmol., 0.10 eq.). The reaction mixture was refluxed under nitrogen atmosphere for 24 hours. The reaction was quenched with water, tetrahydrofuran was evaporated off and resulting aqueous residue was extracted with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate, and concentrated under reduced pressure to give 7-methyl-3-tetrahydropyran-2-yl-benzimidazole-5- carboxylic acid (Intermediate I-2) as a brown solid. LC/MS (Method A) retention time = 0.86 min; [M+H]
+ = 261 Step 2: Preparation of 3-(4-fluoro-3-methoxy-anilino)propanenitrile

To a suspension of copper iodide (0.283 g, 1.49 mmol, 0.07 eq.), potassium tert-butoxide (0.477 g, 4.25 mmol, 0.20 eq.) and 1,3-bis[2,6-bis(1-methylethyl)phenyl]-1H-imidazolium chloride (0.635 g, 1.49 mmol, 0.07 eq.) in toluene (70.00 mL) was added 4-fluoro-3-methoxy-aniline [CAS 64465-53-8] (3.00 g, 21.3 mmol) successively at 25 °C under nitrogen atmosphere. The reaction mixture was allowed to premix for 30min, then a solution of prop-2-enenitrile [CAS 107-13-1] (2.26 g, 42.5 mmol, 2.0 eq.) in toluene (10 mL) was added to the reaction mixture. The resulting reaction mixture was stirred at room temperature for 3 hours before it was quenched with saturated aqueous solution of NH4Cl and extracted with ethyl acetate. The combined organic layers were dried over sodium sulfate, filtered, and concentrated in vacuo. The residue was purified by silica gel column chromatography (petroleum ether/ethyl acetate) to afford 3-(4-fluoro-3-methoxy-anilino)propanenitrile as a brown solid. LC/MS (Method A) retention time = 1.06 min; [M+H]
+ = 195 Step 3: Preparation of N-(2-cyanoethyl)-N-(4-fluoro-3-methoxy-phenyl)-7-methyl-3-tetrahydropyran-2- yl-benzimidazole-5-carboxamide
To a solution of 7-methyl-3-tetrahydropyran-2-yl-benzimidazole-5-carboxylic acid (Intermediate I-2, 400 mg, 1.54 mmol) and 3-(4-fluoro-3-methoxy-anilino)propanenitrile (313 mg, 1.61 mmol, 1.05 eq.) in pyridine (15.0 mL) was added phosphorus(V) oxychloride (707 mg, 4.61 mmol, 3.0 eq.) dropwise at 0 °C. The resulting solution was slowly warmed to room temperature and stirred for 4 hours. Then it was quenched with saturated aqueous NaHCO3 solution and extracted with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (dichloromethane/methanol) to give N-(2-cyanoethyl)-N-(4-fluoro-3-methoxy-phenyl)-7-methyl-3-tetrahydropyran-2-yl-benzimidazole- 5-carboxamide as a sticky oil. LC/MS (Method A) retention time = 1.08 min; [M+H]
+ = 437 Step 4: Preparation of N-(2-cyanoethyl)-N-(4-fluoro-3-methoxy-phenyl)-7-methyl-3H-benzimidazole-5- carboxamide
To a solution of N-(2-cyanoethyl)-N-(4-fluoro-3-methoxy-phenyl)-7-methyl-3-tetrahydropyran-2-yl- benzimidazole-5-carboxamide (170 mg, 0.389 mmol) in ethanol (15.0 mL) was added pyridinium 4- methylbenzenesulfonate (117 mg, 0.467 mmol, 1.20 eq.) under nitrogen atmosphere. The reaction mixture was stirred at 75 °C for 8 hours before the resulting reaction mixture was diluted with water and extracted with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (ethyl acetate/petroleum ether) to give N-(2-cyanoethyl)-N-(4-fluoro-3-methoxy- phenyl)-7-methyl-3H-benzimidazole-5-carboxamide as a brown solid. LC/MS (Method A) retention time = 0.80 min; [M+H]
+ = 353 Step 5: Preparation of methyl N-[5-[6-[2-cyanoethyl-(4-fluoro-3-methoxy-phenyl)carbamoyl]-4-methyl- benzimidazol-1-yl]-2-pyridyl]carbamate (compound 2) To a solution of N-(2-cyanoethyl)-N-(4-fluoro-3-methoxy-phenyl)-7-methyl-3H-benzimidazole-5- carboxamide (110 mg, 0.312 mmol) and methyl N-[5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2-
pyridyl]carbamate [CAS 1073372-02-7] (174 mg, 0.624 mmol, 2.0 eq.) in methanol (10.00 mL) was added copper(I) oxide (134 mg, 0.937 mmol, 3.0 eq.). The reaction mixture was stirred at 35 °C for 24 hours under oxygen atmosphere. Then the mixture was filtered through celite and the filtrate was concentrated under reduced pressure to give the crude which was purified by column chromatography on silica gel (dichloromethane/methanol). It was further purified by trituration in diethylether to afford qualified methyl N-[5-[6-[2-cyanoethyl-(4-fluoro-3-methoxy-phenyl)carbamoyl]-4-methyl-benzimidazol- 1-yl]-2-pyridyl]carbamate as an off-white powder. LC/MS (Method A) retention time = 1.43 min; [M+H]
+ = 503
1H NMR (400 MHz, DMSO-d6, ppm) δ = 10.56 (s, 1H), 8.53 (s, 1H), 8.40 (d, J = 2.7 Hz, 1H), 8.05 (d, J = 8.9 Hz, 1H), 7.73 (dd, J = 8.9, 2.8 Hz, 1H), 7.25 (dd, J = 7.9, 2.5 Hz, 1H), 7.20 (s, 2H), 7.04 (dd, J = 11.3, 8.5 Hz, 1H), 6.69 - 6.58 (m, 1H), 4.08 (t, J = 6.7 Hz, 2H), 3.74 (s, 3H), 3.71 (s, 3H), 2.84 (t, J = 6.7 Hz, 2H), 2.50 (s, 3H) Example 9: Preparation of methyl N-[5-[4-ethyl-6-[(4-fluoro-3-methoxy-phenyl)-methyl- carbamoyl]benzimidazol-1-yl]-2-pyridyl]carbamate (Compound 3)
Step 1: Preparation of methyl 7-vinyl-3H-benzimidazole-5-carboxylate
To a mixture of methyl 7-bromo-3H-benzimidazole-5-carboxylate [CAS 1354756-19-6] (750 mg, 2.94 mmol) and potassium vinyltrifluoroborate [CAS 13682-77-4] (0.591 g, 4.41 mmol, 1.50 eq.) in per-mixed dioxane/water (10:1, 10.0 mL) was added [1,1'-bis(diphenylphosphino)ferrocene] palladium(II) dichlorid (0.215 g, 0.294 mmol, 0.10 eq.) and potassium carbonate (0.813 g, 5.88 mmol, 2.0 eq.). The reaction mixture was stirred at 80 °C for 8 hours under nitrogen atmosphere. Then the resulting mixture was diluted with water and extracted with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (methanol/dichloromethane) to give methyl 7-vinyl-3H- benzimidazole-5-carboxylate as a yellow solid. LC/MS (Method A) retention time = 0.63 min; [M+H]
+ = 203 Step 2: Preparation of methyl 7-ethyl-3H-benzimidazole-5-carboxylate
To a solution of methyl 7-vinyl-3H-benzimidazole-5-carboxylate (0.350 g, 1.73 mmol) in methanol (6.00 mL) was added palladium on carbon (35 mg). The mixture was evacuated and backfilled with hydrogen three times and then charged with hydrogen. The resulting reaction mixture was stirred at room temperature for 5 hours. Then it was filtered through celite and the filtrate was concentrated under reduced pressure to give crude methyl 7-ethyl-3H-benzimidazole-5-carboxylate. LC/MS (Method A) retention time = 0.666 min; [M+H]
+ = 205 Step 3: Preparation of 7-ethyl-3H-benzimidazole-5-carboxylic acid
To a solution of methyl 7-ethyl-3H-benzimidazole-5-carboxylate (0.250 g, 1.22 mmol) in tetrahydrofuran/water (1/1, 5.0 mL) was added lithium hydroxide (0.117 g, 4.90 mmol, 4.0 eq.). The reaction mixture was stirred at 40 °C for 5 hours before the organic solvent was removed under reduced pressure. The aqueous solution was adjusted to pH 2-3 with aqueous 2 N HCl. The formed precipitate was collected by filtration, washed with water and dried to afford 7-ethyl-3H-benzimidazole-5-carboxylic acid as yellow solid. LC/MS (Method A) retention time = 0.49 min; [M+H]
+ = 191 Step 4: Preparation of 7-ethyl-N-(4-fluoro-3-methoxy-phenyl)-N-methyl-3H-benzimidazole-5- carboxamide
To a solution of 4-fluoro-3-methoxy-N-methyl-aniline [CAS 904321-01-3] (0.150 g, 0.967 mmol) and 7- ethyl-3H-benzimidazole-5-carboxylic acid (0.202 g, 1.06 mmol, 1.10 eq.) in pyridine (20.0 mL) was added phosphoryl chloride (0.445 g, 2.90 mmol, 3.0 eq.) dropwise at 0 °C. After addition, the reaction mixture was stirred at room temperature for 2 hours before it was adjusted to pH~7 with NaHCO3
solution. The resulting mixture was diluted with water and extracted with ethyl acetate. The combined organic phases were washed with brine, dried over sodium sulfate, concentrated under reduced pressure and purified by reversed phase chromatography (acetonitrile/water containing 0.1% formic acid) to afford 7-ethyl-N-(4-fluoro-3-methoxy-phenyl)-N-methyl-3H-benzimidazole-5-carboxamide as a yellow solid. LC/MS (Method A) retention time = 0.85 min; [M+H]
+ = 328
1H NMR (400 MHz, DMSO-d
6, ppm) δ = 12.63 (s, 1H), 8.19 (s, 1H), 7.37 (s, 1H), 7.14 - 6.89 (m, 3H), 6.65 (dd, J = 5.4, 3.0 Hz, 1H), 3.67 (d, J = 1.6 Hz, 3H), 3.39 (d, J = 1.6 Hz, 3H), 2.78 (d, J = 6.2 Hz, 2H), 1.18 - 1.02 (m, 3H)

To a solution of 7-ethyl-N-(4-fluoro-3-methoxy-phenyl)-N-methyl-3H-benzimidazole-5-carboxamide (0.100 g, 0.305 mmol) and methyl N-[5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2- pyridyl]carbamate [CAS 1073372-02-7] (0.170 g, 0.611 mmol, 2.0 eq.) in methanol (5.00 mL) was added copper(I) oxide (0.175 g, 1.22 mmol, 4.0 eq.). The reaction mixture was stirred at 35 °C for 8 hours under oxygen atmosphere before it was filtered. The filtrate was concentrated under reduced pressure and the residue was purified by silica gel column chromatography (methanol/dichloromethane). It was further purified by trituration with methyl tert-butyl ether/acetonitrile (1:1) to obtain methyl N-[5-[4-ethyl- 6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]benzimidazol-1-yl]-2-pyridyl]carbamate as a white solid. LC/MS (Method A) retention time = 1.28 min; [M+H]
+ = 478
1H NMR (400 MHz, DMSO-d
6, ppm) δ = 10.55 (s, 1H), 8.52 (s, 1H), 8.45 (d, J = 2.6 Hz, 1H), 8.05 (d, J = 8.8 Hz, 1H), 7.84 (dd, J = 8.8, 2.6 Hz, 1H), 7.32 – 7.27 (m, 1H), 7.17 (dd, J = 7.8, 2.4 Hz, 1H), 7.11 (s, 1H), 7.02 (dd, J = 11.2, 8.6 Hz, 1H), 6.65 (dt, J = 8.4, 3.4 Hz, 1H), 3.74 (s, 3H), 3.67 (s, 3H), 3.37 (s, 3H), 2.89 (q, J = 7.4 Hz, 2H), 1.17 (t, J = 7.4 Hz, 3H)
The reaction vessel was charged with 4-fluoro-3-methoxy-aniline [CAS 64465-53-8] (3.00 g, 21.3 mmol), 2-bromoacetonitrile [CAS 590-17-0] (2.80 g, 23.4 mmol, 1.10 eq.), sodium acetate (2.62 g, 31.9 mmol, 1.50 eq.) and anhydrous ethanol (50 mL). The reaction mixture was heated at 80℃ under nitrogen atmosphere for 7 hours. Then the reaction mixture was concentrated in reduced pressure and the residue was purified by reversed phase chromatography (C18 column, eluting with acetonitrile/water containing 0.1% formic acid) to afford 2-(4-fluoro-3-methoxy-anilino)acetonitrile as a brown solid. LC/MS (Method A) retention time = 1.02 min; [M+H]
+ = 181 Step 2: Preparation of N-(cyanomethyl)-N-(4-fluoro-3-methoxy-phenyl)-7-methyl-3-tetrahydropyran-2- yl-benzimidazole-5-carboxamide

To a solution of 2-(4-fluoro-3-methoxy-anilino)acetonitrile (0.300 g, 1.67 mmol) and 7-methyl-3- tetrahydropyran-2-yl-benzimidazole-5-carboxylic acid (Intermediate I-2, 0.477 g, 1.83 mmol, 1.10 eq.) in pyridine (30.0 mL) was added phosphoryl chloride (0.766 g, 5.00 mmol, 3.0 eq.) dropwise at 0 °C. After addition, the reaction mixture was stirred at room temperature for 2 hours. The aqueous solution was adjusted to pH~7 with NaHCO3 solution and diluted with water. It was extracted with ethyl acetate, the combined organic phases were washed with brine, dried over sodium sulfate, concentrated under reduced pressure. The residue was purified by silica gel column chromatography (methanol/dichloromethane) to give N-(cyanomethyl)-N-(4-fluoro-3-methoxy-phenyl)-7-methyl-3- tetrahydropyran-2-yl-benzimidazole-5-carboxamide as a yellow solid. LC/MS (Method A) retention time = 1.12 min; [M+H]
+ = 423 Step 3: Preparation of N-(cyanomethyl)-N-(4-fluoro-3-methoxy-phenyl)-7-methyl-3H-benzimidazole-5- carboxamide
To a solution of N-(cyanomethyl)-N-(4-fluoro-3-methoxy-phenyl)-7-methyl-3-tetrahydropyran-2-yl- benzimidazole-5-carboxamide (0.300 g, 0.710 mmol) in dioxane (6.00 mL) was added pyridinium 4- methylbenzenesulfonate (0.214 g, 0.852 mmol, 1.20 eq.) under nitrogen atmosphere. The reaction mixture was heated at 75 °C for 3 hours before it was concentrated under reduced pressure. Then the residue was diluted with water and extracted with ethyl acetate. The combined organic layers were washed with NaHCO3, dried over sodium sulfate, and concentrated under reduced pressure. The crude was purified by silica gel column chromatography (methanol/dichloromethane) to afford N- (cyanomethyl)-N-(4-fluoro-3-methoxy-phenyl)-7-methyl-3H-benzimidazole-5-carboxamide as a yellow solid. LC/MS (Method A) retention time = 0.813 min; [M+H]
+ = 339
1H NMR (400 MHz, DMSO-d6, ppm) δ = 12.60 (d, J = 80.1 Hz, 1H), 8.22 (d, J = 7.8 Hz, 1H), 7.43 - 7.24 (m, 2H), 7.19 - 7.04 (m, 2H), 6.63 (d, J = 7.8 Hz, 1H), 4.93 (s, 2H), 3.78 (s, 3H), 2.42 (d, J = 9.2 Hz, 3H) Step 4: Preparation of methyl N-[5-[6-[cyanomethyl-(4-fluoro-3-methoxy-phenyl)carbamoyl]-4-methyl- benzimidazol-1-yl]-2-pyridyl]carbamate (Compound 8) To a solution of N-(cyanomethyl)-N-(4-fluoro-3-methoxy-phenyl)-7-methyl-3H-benzimidazole-5- carboxamide (0.0900 g, 0.266 mmol) and methyl N-[5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2- pyridyl]carbamate [CAS 1073372-02-7] (0.148 g, 0.532 mmol, 2.0 eq.) in methanol (5.00 mL) was added copper(I) oxide (0.152 g, 1.06 mmol, 4.0 eq.). The reaction mixture was stirred at 35 °C for 16 hours under oxygen atmosphere. Then the mixture was filtered, and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (methynol/dichloromethane). It was further purified by trituration with methyl tert-butyl ether/acetonitrile (20:1) to obtain methyl N-[5-[6-[cyanomethyl-(4-fluoro-3-methoxy-phenyl)carbamoyl]-4-methyl- benzimidazol-1-yl]-2-pyridyl]carbamate as a yellow solid. LC/MS (Method A) retention time = 1.132 min; [M+H]
+ = 489
1H NMR (400 MHz, DMSO-d6, ppm) δ = 10.55 (s, 1H), 8.56 (s, 1H), 8.41 (s, 1H), 8.04 (d, J = 9.0 Hz, 1H), 7.75 (d, J = 8.8 Hz, 1H), 7.33 (d, J = 7.6 Hz, 2H), 7.26 (s, 1H), 7.07 (t, J = 9.8 Hz, 1H), 6.63 (d, J = 8.2 Hz, 1H), 4.91 (s, 2H), 3.74 (s, 6H) Example 11: Preparation of methyl N-[5-[6-[(4-cyano-3-methoxy-phenyl)-methyl-carbamoyl]-4- methyl-benzimidazol-1-yl]-2-pyridyl]carbamate (compound 9)
(Compound 9) Step 1: Preparation of N-(4-cyano-3-methoxy-phenyl)-N,7-dimethyl-3H-benzimidazole-5-carboxamide

To a solution of 7-methyl-3H-benzimidazole-5-carboxylic acid [CAS 398452-96-5] (0.400 g, 2.27 mmol) in pyridine (10.00 mL) was added 2-methoxy-4-(methylamino)benzonitrile [CAS 627468-17-1] (0.442 g, 2.72 mmol, 1.20 eq.) at 0 °C under nitrogen atmosphere. After addition, the solution was stirred at 0 °C for 5 minutes. Then phosphoryl chloride (1.04 g, 6.81 mmol, 3.0 eq.) was added dropwise. The resulting reaction mixture was slowly warmed up to room temperature and stirred for 2 hours. Then the resulting mixture was diluted with water and extracted with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (methanol/dichloromethane) to give N-(4-cyano-3- methoxy-phenyl)-N,7-dimethyl-3H-benzimidazole-5-carboxamide as a yellow solid. LC/MS (Method A) retention time = 1.23 min; [M+H]
+ = 321 Step 2: Preparation of methyl N-[5-[6-[(4-cyano-3-methoxy-phenyl)-methyl-carbamoyl]-4-methyl- benzimidazol-1-yl]-2-pyridyl]carbamate (compound 9) To a solution of N-(4-cyano-3-methoxy-phenyl)-N,7-dimethyl-3H-benzimidazole-5-carboxamide (0.330 g, 1.03 mmol) and methyl N-[5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2-pyridyl] carbamate [CAS 1073372-02-7] (0.573 g, 2.06 mmol, 2.0 eq.) in methanol (5.00 mL) was added copper(I) oxide (0.442 g, 3.09 mmol, 3.0 eq.). The reaction mixture was stirred at 40 °C for 48 hours under oxygen atmosphere before it was filtered through Celite. The filtrate was concentrated under reduced pressure to give the crude which was purified by column chromatography on silica gel (methanol/dichloromethane) to afford methyl N-[5-[6-[(4-cyano-3-methoxy-phenyl)-methyl-carbamoyl]-4-methyl-benzimidazol-1-yl]-2-pyridyl] carbamate as a white solid. LC/MS (Method A) retention time = 1.16 min; [M+H]
+ = 471
1H NMR (400 MHz, DMSO-d6, ppm) δ = 10.56 (s, 1H), 8.55 (s, 1H), 8.41 (d, J = 2.3 Hz, 1H), 8.04 (d, J = 9.2 Hz, 1H), 7.76 (dd, J = 8.9, 2.7 Hz, 1H), 7.54 (d, J = 8.3 Hz, 1H), 7.27 (s, 1H), 7.22 (d, J = 1.8 Hz, 1H), 7.18 (s, 1H), 6.79 (dd, J = 8.3, 1.9 Hz, 1H), 3.75 (s, 3H), 3.74 (s, 3H), 3.42 (s, 3H), 2.51 (s, 3H)
Example 12: Preparation of methyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-(2- methoxyethyl)carbamoyl]-4-methyl-benzimidazol-1-yl]-2-pyridyl]carbamate (Compound 13)
(Compound 13) Step 1: Preparation of 4-fluoro-3-methoxy-N-(2-methoxyethyl)aniline
To a mixture of 4-bromo-1-fluoro-2-methoxy-benzene [CAS 103291-07-25] (3.00 g, 14.6 mmol) in toluene (60.0 mL) was added 2-methoxyethanamine [CAS 109-85-3] (1.43 g, 19.0 mmol, 1.30 eq.), potassium tert-butoxide (4.10 g, 36.6 mmol, 2.50 eq.), Xantphos (0.423 g, 0.732 mmol, 0.05 eq.) and tris(dibenzylideneacetone)dipalladium(0) (0.67 g, 0.732 mmol, 0.05 eq.). The reaction mixture was stirred at 80 °C for 4 hours under nitrogen atmosphere, then it was diluted with ethyl acetate, washed with water and brine, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (dichloromethane/methanol) to obtain 4-fluoro-3-methoxy-N-(2-methoxyethyl)aniline as a brown oil. LC/MS (Method A) retention time = 1.05 min; [M+H]
+ = 200 Step 2: Preparation of N-(4-fluoro-3-methoxy-phenyl)-N-(2-methoxyethyl)-7-methyl-3H-benzimidazole- 5-carboxamide

To a solution of 4-fluoro-3-methoxy-N-(2-methoxyethyl)aniline (0.600 g, 3.01 mmol) and 7-methyl-3H- benzimidazole-5-carboxylic acid [CAS 398452-96-5] (0.584 g, 3.31 mmol, 1.10 eq.) in pyridine (20.0 mL) was added phosphoryl chloride (1.39 g, 9.04 mmol, 3.0 eq.) dropwise at 0 °C. After addition, the solution was stirred at room temperature. for 2 hours before it was adjusted to pH 7 with NaHCO3 solution. The resulting mixture was diluted with water and extracted with ethyl acetate. The combined
organic phases were washed with brine, dried over sodium sulfate and concentrated under reduced pressure. The crude was purified by reversed phase chromatography (C18 column, eluting with acetonitrile/water containing 0.1% formic acid) to provide N-(4-fluoro-3-methoxy-phenyl)-N-(2- methoxyethyl)-7-methyl-3H-benzimidazole-5-carboxamide as a white solid. LC/MS (Method A) retention time = 0.83 min; [M+H]
+ = 358
1H NMR (400 MHz, DMSO-d6, ppm) δ = 12.59 (s, 1H), 8.18 (s, 1H), 7.25 (s, 1H), 7.11 (dd, J = 7.8, 2.4 Hz, 1H), 7.01 (dd, J = 10.0, 7.4 Hz, 2H), 6.69 - 6.59 (m, 1H), 3.98 (t, J = 5.8 Hz, 2H), 3.71 (s, 3H), 3.52 (t, J = 5.8 Hz, 2H), 3.26 (s, 3H), 2.41 (s, 3H) Step 3: Preparation of methyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-(2-methoxyethyl)carbamoyl]-4- methyl-benzimidazol-1-yl]-2-pyridyl]carbamate (compound 13) To a solution of N-(4-fluoro-3-methoxy-phenyl)-N-(2-methoxyethyl)-7-methyl-3H-benzimidazole-5- carboxamide (0.120 g, 0.336 mmol) and methyl N-[5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2- pyridyl]carbamate [CAS 1073372-02-7] (0.187 g, 0.672 mmol, 2.0 eq.) in methanol (5.00 mL) was added copper(I) oxide (0.192 g, 1.34 mmol, 4.0 eq.). The reaction mixture was stirred at 35 °C for 8 hours under oxygen atmosphere before it was filtered. The filtrate was concentrated under reduced pressure and the residue was purified by silica gel column chromatography (methanol/dichloromethane). It was further purified by trituration with acetonitrile to obtain methyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-(2- methoxyethyl)carbamoyl]-4-methyl-benzimidazol-1-yl]-2-pyridyl]carbamate as an off-white solid. LC/MS (Method A) retention time = 1.14 min; [M+H]
+ = 508
1H NMR (400 MHz, DMSO-d6, ppm) δ = 10.55 (s, 1H), 8.52 (s, 1H), 8.41 (d, J = 2.4 Hz, 1H), 8.05 (d, J = 8.8 Hz, 1H), 7.75 (d, J = 7.4 Hz, 1H), 7.23 - 7.14 (m, 3H), 7.01 (dd, J = 11.0, 8.8 Hz, 1H), 6.60 (d, J = 8.0 Hz, 1H), 3.97 (t, J = 5.8 Hz, 2H), 3.74 (s, 3H), 3.70 (s, 3H), 3.51 (t, J = 5.6 Hz, 2H), 3.23 (s, 3H), 2.50 (s, 3H) Example 13: Preparation of methyl N-[5-[6-[ethyl-(4-fluoro-3-methoxy-phenyl)carbamoyl]-4- (methoxymethyl)benzimidazol-1-yl]-2-pyridyl]carbamate (Compound 5)

(Compound 5) Step 1: Preparation of N-ethyl-N-(4-fluoro-3-methoxy-phenyl)-7-(methoxymethyl)-3-(2- trimethylsilylethoxymethyl)benzimidazole-5-carboxamide
To a solution of N-ethyl-4-fluoro-3-methoxy-aniline (0.300 g, 1.77 mmol) and 7-(methoxymethyl)-3-(2- trimethylsilylethoxymethyl)benzimidazole-5-carboxylic acid (Intermediate I-1, 0.597 g, 1.77 mmol) in pyridine (6.00 mL) was propylphosphonic anhydride (1.69 g, 5.32 mmol). The mixture was stirred at 80 °C for 2 hours. The aqueous solution was adjusted to pH 7 with saturated aqueous NaHCO3 solution. The resulting mixture was diluted with water and extracted with ethyl acetate. The combined organic phases were washed with brine, dried over sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (MeOH/dichloromethane) to give N-ethyl-N-(4-fluoro-3-methoxy-phenyl)-7-(methoxymethyl)-3-(2-trimethylsilylethoxymethyl) benzimidazole-5-carboxamide as a yellow solid. LC/MS (Method A) retention time = 1.15 min; [M+H]
+ = 488 Step 2: Preparation of N-ethyl-N-(4-fluoro-3-methoxy-phenyl)-7-(methoxymethyl)-3H-benzimidazole-5- carboxamide
N-ethyl-N-(4-fluoro-3-methoxy-phenyl)-7-(methoxymethyl)-3-(2-trimethylsilylethoxymethyl) benzimidazole-5-carboxamide (0.300 g, 0.615 mmol) was added to a stirred mixture of trifluoroacetic acide/dichloromethane (1:1, 6.00 mL). the reaction stirred at 25 °C for 8 hours. Then the resulting mixture was diluted with water and extracted with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate, filtered and concentrated under vacuum. The residue was purified by silica gel column chromatography (MeOH/dichloromethane) to give N-ethyl-N-(4-fluoro-3- methoxy-phenyl)-7-(methoxymethyl)-3H-benzimidazole-5-carboxamide as a yellow solid. LC/MS (Method A) retention time = 0.89 min; [M+H]
+ = 358
Step 3: Preparation of methyl N-[5-[6-[ethyl-(4-fluoro-3-methoxy-phenyl)carbamoyl]-4- (methoxymethyl)benzimidazol-1-yl]-2-pyridyl]carbamate (Compound 5) To a solution of N-ethyl-N-(4-fluoro-3-methoxy-phenyl)-7-(methoxymethyl)-3H-benzimidazole-5- carboxamide (0.180 g, 0.504 mmol) and methyl N-[5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2- pyridyl]carbamate (0.280 g, 1.01 mmol) in MeOH (5.00 mL) was added cupriooxycopper (0.216 g, 1.51 mmol). The mixture was stirred at 40 °C for 48 hours under oxygen atmosphere. The mixture was then filtered through celite and the filtrate was concentrated under reduced pressure. The crude residue was purified by column chromatography on silica gel (dichloromethane/MeOH), then further by SFC (Column: Torus 2-PIC 20*250 mm, 5um; Mobile phase: CO2/MeOH (0.1% NH3) = 60/40) to give methyl N-[5-[6-[ethyl-(4-fluoro-3-methoxy-phenyl)carbamoyl]-4-(methoxymethyl)benzimidazol-1-yl]-2- pyridyl]carbamate. LC/MS (Method A) retention time = 1.15 min; [M+H]
+ = 508
1H NMR (400 MHz, DMSO-d6, ppm) δ = 10.56 (s, 1H), 8.55 (s, 1H), 8.45 (d, J = 2.5 Hz, 1H), 8.06 (d, J = 8.9 Hz, 1H), 7.83 (dd, J = 8.9, 2.2 Hz, 1H), 7.39 (s, 1H), 7.31 (s, 1H), 7.19 (dd, J = 7.8, 2.1 Hz, 1H), 7.00 (dd, J = 11.2, 8.7 Hz, 1H), 6.60 (d, J = 8.2 Hz, 1H), 4.74 (s, 2H), 4.74 (s, 2H), 3.86 (dd, J = 14.2, 7.1 Hz, 3H), 3.74 (s, 3H), 3.70 (s, 3H), 3.21 (s, 3H), 1.12 (t, J = 7.0 Hz, 3H). Example 14: Preparation of methyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)- (methoxymethyl)carbamoyl]-4-methyl-benzimidazol-1-yl]-2-pyridyl]carbamate (Compound 23)
(Compound 23) Step 1: Preparation of 2-[(6-bromo-4-methyl-benzimidazol-1-yl)methoxy]ethyl-trimethyl-silane
6-bromo-4-methyl-1H-benzimidazole (CAS 255064-10-9, 2.00 g, 9.48 mmol) was dissolved in tetrahydrofuran (30 mL) and the solution was cooled down to 0 °C. Sodium hydride (0.454 g, 11.37 mmol, 1.2 eq., 60 mass% in oil) was added. The reaction mixture was stirred at 0 °C for 30 min.2-
(trimethylsilyl)ethoxymethyl chloride (2.02 mL, 11.4 mmol, 1.20 eq.) was then added at 0 °C. the reaction mixture was stirred at room temperature for 12 hours. The reaction was then quenched by addition of saturated NH4Cl solution, the aqueous layer was extracted with ethyl acetate, the combined organic layers were washed with water then brine, dried over sodium sulfate, filtered and concentrated under reduced pressure. The crude residue was purified by column chromatography on silica gel (hexane/ethylacetate) to give 2-[(6-bromo-4-methyl-benzimidazol-1-yl)methoxy]ethyl-trimethyl-silane.
1H NMR (400 MHz, CDCl
3, ppm) δ = -0.04 (d, J = 3.50 Hz, 18 H), -0.02 (br s, 1 H), -0.01 (br s, 1 H), 0.86 - 0.96 (m, 5 H), 2.64 - 2.71 (m, 7 H), 3.49 (td, J = 8.13, 5.13 Hz, 4 H), 5.48 (s, 2 H), 5.57 (s, 2 H), 7.21 (s, 1 H), 7.25 (dd, J = 1.63, 0.75 Hz, 1 H), 7.27 (s, 1 H), 7.54 (d, J = 1.13 Hz, 1 H), 7.79 (d, J = 1.25 Hz, 1 H), 7.86 (s, 1 H), 7.92 (s, 1 H) Step 2: Preparation of N-(4-fluoro-3-methoxy-phenyl)-7-methyl-3-(2- trimethylsilylethoxymethyl)benzimidazole-5-carboxamide
To a solution of 2-[(6-bromo-4-methyl-benzimidazol-1-yl)methoxy]ethyl-trimethyl-silane (1.00 g, 2.93 mmol) in toluene (10.0 mL) were added 4-fluoro-3-methoxyaniline (0.418 g, 2.96 mmol, 1.01 eq.), bis(benzonitrile)palladium chloride (0.293 mmol, 0.10 eq.), 4,5-bis(diphenylphosphino)-9,9- dimethylxanthene (0.118 g, 0.293 mmol, 0.10 equiv) and triethylamine (0.62 mL, 4.4 mmol, 1.5 eq.) The vial was flushed with nitrogen then CO gas and then filled with 10 bar CO gas. The reaction was heated at 100 °C for 6 hours. The reaction mixture was filtered on celite, and the filtrate was concentrated under reduced pressure. The crude residue was purified by column chromatography on silica gel (cyclohexane/ethylacetate) to afford N-(4-fluoro-3-methoxy-phenyl)-7-methyl-3-(2- trimethylsilylethoxymethyl)benzimidazole-5-carboxamide as a brown solid.
1H NMR (400 MHz, CDCl3, ppm) δ = -0.03 - 0.01 (m, 1 H), 0.87 - 0.97 (m, 2 H), 1.26 (br d, J = 2.13 Hz, 1 H), 2.05 (s, 1 H), 2.74 (s, 3 H), 3.49 - 3.58 (m, 2 H), 3.94 (s, 3 H), 5.58 (s, 2 H), 6.93 - 7.01 (m, 1 H), 7.07 (dd, J = 10.82, 8.69 Hz, 1 H), 7.27 (s, 1 H), 7.59 (s, 1 H), 7.71 (dd, J = 7.75, 2.38 Hz, 1 H), 7.95 - 8.02 (m, 2 H), 8.10 (s, 1 H). Step 3: preparation of N-(4-fluoro-3-methoxy-phenyl)-N-(methoxymethyl)-7-methyl-3-(2- trimethylsilylethoxymethyl)benzimidazole-5-carboxamide
A solution of N-(4-fluoro-3-methoxy-phenyl)-7-methyl-3-(2-trimethylsilylethoxymethyl)benzimidazole-5- carboxamide (100 mg, 0.232 mmol) in tetrahydrofuran (1.0 mL) was cooled down to 0 °C. Sodium hydride (18.6 mg, 0.466 mmol, 2.00 equiv, 60 mass% in oil) was added portionwise. After 30 min of stirring at 0 ºC, chloromethyl methyl ether (0.026 mL, 0.349 mmol, 1.50 eq.) was added to the reaction mixture slowly. The resulting reaction mixture was stirred at room temperature overnight. The reaction was quenched by addition of saturated aqueous ammonium chloride solution at 0 °C. The aqueous layer was extracted with ethyl acetate, the combined organic layers were washed with water then brine, dried over sodium sulfate, filtered and concentrated under reduced pressure.
1H NMR (400 MHz, CDCl3, ppm) δ = -0.06 - 0.01 (m, 9 H), 0.80 - 0.93 (m, 2 H), 2.60 (s, 3 H), 3.34 - 3.48 (m, 2 H), 3.51 (s, 3 H), 3.73 - 3.76 (m, 3 H), 5.25 (s, 2 H), 5.42 (s, 2 H), 6.65 - 6.75 (m, 1 H), 6.76 – 6.78 (m, 1 H), 6.78 – 7.00 (m, 1 H), 7.29 (s, 1 H), 7.44 (s, 1 H) 8.00 (s, 1 H). Step 4: preparation of N-(4-fluoro-3-methoxy-phenyl)-N-(methoxymethyl)-7-methyl-3H-benzimidazole- 5-carboxamide (Intermediate I-3)

(Intermediate I-3) Tetramethylethylenediamine (0.095 mL, 0.633 mmol, 3.00 equiv) and tetrabutylammonium fluoride (1 M in tetrahydrofuran, 0.633 mL, 0.633 mmol, 3.00 eq.) were added to a solution of N-(4-fluoro-3-methoxy- phenyl)-N-(methoxymethyl)-7-methyl-3-(2-trimethylsilylethoxymethyl)benzimidazole-5-carboxamide (0.100 g, 0.211 mmol, 1.00 eq.) in dimethylformamide (1.00 mL). The resulting solution was stirred at 45 °C for 12 hours. the reaction was quenched by addition of water. The aqueous layer was extracted ethyl acetate and the combined organic layers were washed with brine, dried over sodium sulfate, filtered, and concentrated under reduced pressure to afford.N-(4-fluoro-3-methoxy-phenyl)-N- (methoxymethyl)-7-methyl-3H-benzimidazole-5-carboxamide (Intermediate I-3) as brown solid.
1H NMR (400 MHz, CDCl3, ppm) δ = 2.55 (s, 3 H), 3.54 (s, 3 H), 3.79 (s, 3 H), 5.29 (s, 2 H) 6.76 (dt, J = 8.44, 3.10 Hz, 1 H), 6.86 (dd, J = 7.50, 2.38 Hz, 1 H), 6.97 (dd, J = 10.82, 8.57 Hz, 1 H), 7.33 (s, 1 H), 7.61 (s, 1 H), 8.07 (s, 1 H).
Step 5: Preparation of methyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-(methoxymethyl)carbamoyl]-4- methyl-benzimidazol-1-yl]-2-pyridyl]carbamate (Compound 23) Boric acid (0.036 g, 0.58 mmol, 2. eq.) and copper(II) acetate (0.079 g, 0.44 mmol, 1.5 eq.) were added to a solution of N-(4-fluoro-3-methoxy-phenyl)-N-(methoxymethyl)-7-methyl-3H-benzimidazole-5- carboxamide (Intermediate I-3, 0.100 g, 0.291 mmol) in acetonitrile (7.6 mL). Then, N,N- diisopropylethylamine (0.104 mL, 0.582 mmol, 2.00 eq.) was added followed by methyl N-[5-(4,4,5,5- tetramethyl-1,3,2-dioxaborolan-2-yl)-2-pyridyl]carbamate (0.162 g, 0.582 mmol, 2.00 eq.) The reaction was stirred at 65 °C for 12 hours. The reaction mixture was poured into water. The aqueous layer was extracted with ethyl acetate, the combined organic layers were washed with water then brine, dried over sodium sulfate, filtered and concentrated under reduced pressure. The crude residue was purified by reverse phase chromatography (acetonitrile/water) to afford methyl N-[5-[6-[(4-fluoro-3-methoxy- phenyl)-(methoxymethyl)carbamoyl]-4-methyl-benzimidazol-1-yl]-2-pyridyl]carbamate (Compound 23) as light brown solid. LC/MS (Method B) retention time = 1.01 min; [M+H]
+ = 494
1H NMR (400 MHz, DMSO-d
6, ppm) δ = 2.52 (s, 3 H), 3.31 (s, 3 H), 3.71 (s, 3 H), 3.74 (s, 3 H), 5.14 (s, 2 H), 6.67 - 6.71 (m, 1 H), 7.00 - 7.09 (m, 1 H), 7.21 (dd, J = 7.89, 2.38 Hz, 1 H), 7.26 (s, 1 H), 7.33 (br s, 1 H), 7.76 - 7.85 (m, 1 H), 8.06 (d, J = 8.93 Hz, 1 H), 8.44 (d, J = 2.32 Hz, 1 H), 8.56 (s, 1 H), 10.57 (s, 1 H). Example 15: Preparation of 3-(6-acetamido-3-pyridyl)-N-(4-fluoro-3-methoxy-phenyl)-N- (methoxymethyl)-7-methyl-benzimidazole-5-carboxamide (Compound 94)
(Compound 94) Step 1: Preparation of 3-(6-amino-3-pyridyl)-N-(4-fluoro-3-methoxy-phenyl)-N-(methoxymethyl)-7- methyl-benzimidazole-5-carboxamide

Boric acid (0.069 g, 1.11 mmol, 2.0 eq.) and copper(II) acetate (0.151 g, 0.830 mmol, 1.50 eq.) were added to a solution of N-(4-fluoro-3-methoxy-phenyl)-N-(methoxymethyl)-7-methyl-3H-benzimidazole- 5-carboxamide (Intermediate I-3, 0.190 g, 0.553 mmol) in acetonitrile (14.4 mL). Then, N,N- diisopropylethylamine (0.197 mL, 1.107 mmol, 2.00 eq.) was added followed by methyl N-[5-(4,4,5,5- tetramethyl-1,3,2-dioxaborolan-2-yl)-2-pyridyl]carbamate (0.146 g, 0.664 mmol, 1.20 eq.). The reaction mixture was purged with oxygen for 10 minutes. The reaction was stirred at room temperature for 12 hours. The reaction mixture was poured into water. The aqueous layer was extracted with ethyl acetate, the combined organic layers were washed with water then brine, dried over sodium sulfate, filtered and concentrated under reduced pressure. The crude residue was purified by flash chromatography on silica gel (cyclohexane/ethyl acetate) to afford 3-(6-amino-3-pyridyl)-N-(4-fluoro-3-methoxy-phenyl)-N- (methoxymethyl)-7-methyl-benzimidazole-5-carboxamide as a gum.
1H NMR (400 MHz, DMSO-d6, ppm) δ = 3.32 (s, 3 H), 3.72 (s, 3 H), 5.14 (s, 2 H), 6.41 (s, 2 H), 6.60 (d, J = 8.88 Hz, 1 H), 6.63 - 6.68 (m, 1 H), 7.05 (dd, J = 11.19, 8.57 Hz, 1 H), 7.16 – 7.25 (m, 3 H), 7.26 - 7.34 (m, 1 H), 7.97 (d, J = 2.50 Hz, 1 H), 8.39 (s, 1 H). Step 2: Preparation of 3-(6-acetamido-3-pyridyl)-N-(4-fluoro-3-methoxy-phenyl)-N-(methoxymethyl)-7- methyl-benzimidazole-5-carboxamide (Compound 94) Pyridine (0.015 mL, 0.184 mmol, 2.00 eq.) and dimethylaminopyridine (5.8 mg, 0.046 mmol, 0.50 eq.) were added to a solution of 3-(6-amino-3-pyridyl)-N-(4-fluoro-3-methoxy-phenyl)-N-(methoxymethyl)-7- methyl-benzimidazole-5-carboxamide (40.0 mg, 0.0919 mmol) in dichloromethane (0.40 mL). Acetyl chloride (10.8 mg, 0.138 mmol, 1.50 eq.) was added portionwise. The mixture was stirred at room temperature for 20 hours. The reaction was then quenched by addition of saturated aqueous sodium bicarbonate solution. The aqueous layer was extracted with ethyl acetate. The combined organic layers were dried over sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified by flash chromatography over silica ge (cyclohexane/ethyl acetate) to afford 3-(6-acetamido-3- pyridyl)-N-(4-fluoro-3-methoxy-phenyl)-N-(methoxymethyl)-7-methyl-benzimidazole-5-carboxamide (Compound 94) as a gum. LC/MS (Method B) retention time = 0.97 min; [M+H]
+ = 478
1H NMR (400 MHz, DMSO-d6, ppm) δ = 2.16 (s, 3 H), 2.52 (s, 3 H), 3.32 (s, 3 H), 3.70 (s, 2 H), 5.14 (s, 2 H), 6.67 (br d, J = 8.25 Hz, 1 H), 7.04 (dd, J = 11.19, 8.57 Hz, 1 H), 7.19 (dd, J = 7.82, 2.44 Hz, 1 H), 7.25 (s, 1 H), 7.32 (br s, 1 H), 7.80 (br d, J = 6.75 Hz, 1 H), 8.29 (d, J = 8.88 Hz, 1 H), 8.47 (d, J = 2.50 Hz, 1 H), 8.56 (s, 1 H) 10.81 (s, 1 H) Example 16: Preparation of methyl N-[5-[2-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]-6- methyl-purin-9-yl]-2-pyridyl]carbamate (Compound 32)
(Compound 32) Step Preparation of 6-methyl-9H-purine-2-carboxylic acid

To a solution of methyl 6-methyl-9H-purine-2-carboxylate (CAS 62134-45-6, 2.50 g, 13.0 mmol) in THF/H2O (30.0 mL, 1:1) was added lithium hydroxide (0.623 g, 26.0 mmol, 2.00 eq.). The mixture was stirred at room temperature for 1 hour. Then the organic solvent was removed under reduced pressure. The aqueous solution was adjusted to pH 2-3 with 2 N HCl. The precipitate was collected by filtration, washed with water and lyophilized to afford 6-methyl-9H-purine-2-carboxylic acid as yellow solid. LC/MS (Method A) retention time = 0.16 min; [M+H]
+ = 179 Step 2: Preparation of N-(4-fluoro-3-methoxy-phenyl)-N,6-dimethyl-9H-purine-2-carboxamide
To a solution of 6-methyl-9H-purine-2-carboxylic acid (0.700 g, 3.93 mmol) and 4-fluoro-3-methoxy-N- methyl-aniline (0.671 g, 4.32 mmol, 1.10 eq.) in pyridine (14.00 mL) was added propanephosphonic acid anhydride (50.0 %, 3.75 g, 5.89 mmol, 1.50 eq.). The mixture was stirred at 70 °C for 2 hours. Then the resulting mixture was diluted with water and the aqueous layer was extracted with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate, and concentrated under vacuum. The residue was purified by silica gel column chromatography (MeOH/dichloromethane) to give N-(4-fluoro-3-methoxy-phenyl)-N,6-dimethyl-9H-purine-2-carboxamide as a yellow solid. LC/MS (Method A) retention time = 0.72 min; [M+H]
+ = 315
Step 3: Preparation of methyl N-[5-[2-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]-6-methyl-purin- 9-yl]-2-pyridyl]carbamate (Compound 32) To a mixture of N-(4-fluoro-3-methoxy-phenyl)-N,6-dimethyl-9H-purine-2-carboxamide (0.400 g, 1.27 mmol) and methyl N-[5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2-pyridyl]carbamate (0.459 g, 1.65 mmol, 1.30 eq.) in acetonitrile (20.0 mL) was added copper diacetate (0.346 g, 1.90 mmol, 1.50 eq.), boric acid (0.157 g, 2.54 mmol, 2.00 eq.) and N-ethyl-N-isopropyl-propan-2-amine (0.492 g, 3.81 mmol, 3.00 eq.). The mixture was stirred at 50 °C for 10 hours under O2 atmosphere. The mixture was filtered through celite. The filtrate was concentrated under vacuum. The crude residue was purified by column chromatography on silica gel (MeOH/dichloromethane) then further purified by reverse-phase chromatography (MeCN/H2O containing 0.1% NH3.H2O) to afford methyl N-[5-[2-[(4-fluoro-3-methoxy- phenyl)-methyl-carbamoyl]-6-methyl-purin-9-yl]-2-pyridyl] carbamate as a yellow solid. LC/MS (Method A) retention time = 1.33 min; [M+H]
+ = 466.3
1H NMR (400 MHz, DMSO-d6, ppm) δ = 10.54 (s, 1 H), 8.91 (s, 1 H), 8.55 (s, 1 H), 8.04 (d, J = 8.9 Hz, 1 H), 7.96 (d, J = 8.2 Hz, 1 H), 7.08 (d, J = 6.5 Hz, 1 H), 6.99-6.95 (m, 1 H), 6.63 (s, 1 H), 3.74 (s, 3 H), 3.66 (s, 3 H), 3.42 (s, 3 H), 2.70 (s, 3 H). Example 17: Preparation of methyl N-[5-[5-[(4-fluoro-3-methoxy-phenyl)-methyl- carbamoyl]imidazo[4,5-b]pyridin-3-yl]-2-pyridyl]carbamate (Compound 12)
(Compound 12) Step 1: Preparation of N-(4-fluoro-3-methoxy-phenyl)-N-methyl-3H-imidazo[4,5-b]pyridine-5- carboxamide
To a solution of 3H-imidazo[4,5-b]pyridine-5-carboxylic acid (CAS 1019108-05-4, 0.410 g, 2.51 mmol, 1.32 eq.) and 4-fluoro-3-methoxy-N-methyl-aniline (0.300 g, 1.93 mmol) in pyridine (6.00 mL) was added propanephosphonic acid anhydride (50.0 %, 3.69 g, 5.80 mmol, 3.00 eq.). The mixture was stirred at 60 °C for 4 hours. Then the resulting mixture was diluted with water and extracted with ethyl acetate. The
combined organic layers were washed with brine, dried over sodium sulfate, and concentrated under vacuum. The residue was purified by silica gel column chromatography (MeOH/dichloromethane) to give N-(4-fluoro-3-methoxy-phenyl)-N-methyl-3H-imidazo[4,5-b]pyridine-5-carboxamide as a yellow solid.
1H NMR (400 MHz, DMSO-d6, ppm) δ = 8.48 (s, 1 H), 7.98 (d, J = 7.8 Hz, 1H), 7.39 (d, J = 8.1 Hz, 1H), 7.07 - 6.94 (m, 2 H), 6.67 (s, 1 H), 3.67 (s, 3 H), 3.41 (s, 3 H). Step 2: Preparation of methyl N-[5-[5-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]imidazo[4,5- b]pyridin-3-yl]-2-pyridyl]carbamate (Compound 12) To a solution of N-(4-fluoro-3-methoxy-phenyl)-N-methyl-3H-imidazo[4,5-b] pyridine-5-carboxamide (0.280 g, 0.932 mmol) and methyl N-[5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2-pyridyl] carbamate (0.389 g, 1.40 mmol, 1.50 eq.) in MeOH (5.00 mL) was added cupriooxycopper (0.400 g, 2.80 mmol, 3.00 eq.). The mixture was stirred at 40 °C for 32 hours under O2. The mixture was filtered through celite. The filtrate was concentrated under vacuum to give the crude which was purified by column chromatography on silica gel (MeOH/dichloromethane) then further purified by trituration (methyl tert-butyl methyl ether/acetonitrile, 10:1) to afford methyl N-[5-[5-[(4-fluoro-3-methoxy-phenyl)-methyl- carbamoyl]imidazo[4,5-b] pyridin-3-yl]-2-pyridyl] carbamate as a yellow solid. LC/MS (Method A) retention time = 2.31 min; [M+H]
+ = 451.3
1H NMR (400 MHz, DMSO-d6, ppm) δ = 10.48 (s, 1 H), 8.88 (s, 1 H), 8.48 (s, 1 H), 8.24 (d, J = 8.3 Hz, 1 H), 7.99 (d, J = 9.0 Hz, 1 H), 7.69 (d, J = 8.3 Hz, 2 H), 7.12 (d, J = 5.6 Hz, 1 H), 6.95 (t, J = 9.6 Hz, 1 H), 6.55 (s, 1 H), 3.74 (s, 3 H), 3.69 (s, 3 H), 3.42 (s, 3 H). Example 18: Preparation of methyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-methyl- carbamoyl]imidazo[4,5-b]pyridin-1-yl]-2-pyridyl]carbamate (Compound 37)

(Compound 37) Step 1: Preparation of N-(4-fluoro-3-methoxy-phenyl)-N-methyl-1H-imidazo[4,5-b]pyridine-6- carboxamide
To a solution of 1H-imidazo[4,5-b] pyridine-6-carboxylic acid (CAS 24638-31-1, 0.400 g, 2.45 mmol) and 4-fluoro-3-methoxy-N-methyl-aniline (0.457 g, 2.94 mmol, 1.18 eq.) in pyridine (6.00 mL) was added propanephosphonic acid anhydride (50.0 %, 4.68 g, 7.36 mmol, 2.50 equiv). The mixture was stirred at 80°C for 2 hours. The reaction was quenched by addition of water. The aqueous layer was extracted with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate, and concentrated under vacuum. The residue was purified by silica gel column chromatography (MeOH/dichloromethane) to give N-(4-fluoro-3-methoxy-phenyl)-N-methyl-1H-imidazo[4,5-b] pyridine- 6-carboxamide as a yellow solid. LC/MS (Method A) retention time = 1.08 min; [M+H]
+ = 301.2
1H NMR (400 MHz, DMSO-d6, ppm) δ = 8.48 (s, 1 H), 8.29 (s, 1 H), 7.91 (s, 1 H), 7.21 (dd, J = 7.8, 2.2 Hz, 1 H), 7.05 (dd, J = 11.2, 8.7 Hz, 1 H), 6.73-6.71 (m, 1 H), 3.71 (s, 3 H), 3.41 (s, 3 H). Step 2: Preparation of methyl N-[5-[6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]imidazo[4,5- b]pyridin-1-yl]-2-pyridyl]carbamate (Compound 37) To a solution of N-(4-fluoro-3-methoxy-phenyl)-N-methyl-1H-imidazo[4,5-b] pyridine-6-carboxamide (0.360 g, 1.20 mmol) and methyl N-[5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2-pyridyl] carbamate (0.500 g, 1.80 mmol, 1.50 eq.) in methanol (6.00 mL) was added cupriooxycopper (0.515 g, 3.60 mmol, 3.00 eq.). The mixture was stirred at 40 °C for 36 hours under O2 atmosphere. The mixture was filtered through celite. The filtrate was concentrated under vacuum. The crude residue was purified by column chromatography on silica gel MeOH/dichloromethane) then further purified by trituration (methyl tertbutyl methyl ether/acetonitrile, 10:1) to afford qualified methyl N-[5-[6-[(4-fluoro-3-methoxy- phenyl)-methyl-carbamoyl]imidazo[4,5-b] pyridin-1-yl]-2-pyridyl] carbamate as a white solid. LC/MS (Method A) retention time = 2.31 min; [M+H]
+ = 451.3
1H NMR (400 MHz, DMSO-d6, ppm) δ = 10.59 (s, 1 H), 8.87 (s, 1 H), 8.53 (d, J = 2.5 Hz, 1 H), 8.41 (d, J = 1.3 Hz, 1 H), 8.06 (d, J = 8.9 Hz, 1 H), 7.97-7.93 (m, 2 H), 7.26 (dd, J = 7.8, 2.4 Hz, 1 H), 7.04 (dd, J = 11.2, 8.6 Hz, 1 H), 6.76 - 6.74 (m, 1 H), 3.74 (s, 3 H), 3.70 (s, 3 H), 3.40 (s, 3 H). Example 20: Preparation of methyl N-[5-[6-[(4-fluorophenyl)-methyl-carbamoyl]imidazo[4,5- c]pyridin-1-yl]-2-pyridyl]carbamate (Compound 42)
(Compound 42) Step 1: Preparation of methyl N-[5-[(2-chloro-5-nitro-4-pyridyl)amino]-2-pyridyl]carbamate.

A solution of 2,4-dichloro-5-nitro-pyridine (7.00 g, 34.5 mmol) and methyl N-(5-amino-2- pyridyl)carbamate (6.49 g, 36.9 mmol, 1.10 eq.) in EtOH (100 mL) was treated with triethylamine (14.1 mL, 103 mmol, 3.00 eq.) and stirred at 100 °C for 6 hours. The reaction mixture was then concentrated under reduced pressure, diluted with water and extracted with ethyl acetate. The combined organic layers were dried over sodium sulfate, filtered and concentrated. The crude residue was purified by column chromatography over silica gel (n-hexane/ethyl acetate) to afford methyl N-[5-[(2-chloro-5-nitro- 4-pyridyl)amino]-2-pyridyl]carbamate.
1H NMR (400 MHz, DMSO-d6, ppm) δ = 10.39 (s, 1 H), 9.95 (s, 1 H), 9.46 - 9.73 (br s, 1 H), 8.98 (s, 1 H), 8.27 (m, 1 H), 7.95 (m, 1 H), 7.80 (m.1 H), 6.71 (s, 1 H), 3.70 (s, 3 H) Step 2: Preparation of methyl N-[5-[(5-amino-2-chloro-4-pyridyl)amino]-2-pyridyl]carbamate
Iron (9.01 g, 161 mmol, 5.00 eq.) was added to a solution of methyl N-[5-[(2-chloro-5-nitro-4- pyridyl)amino]-2-pyridyl]carbamate (11.0 g, 32.3 mmol) in ethanol (1000 mL) and H2O (120 mL), followed by ammonium chloride (17.3 g, 323 mmol, 10.0eq.). The reaction mixture was stirred at 100
°C for 6 hours. The reaction mixture was filtered on celite and the cake was washed with hot ethanol and the filtrate was concentrated under reduced pressure. Water was added to the residue, and the precipitate was filtered off, washed with water and methanol, dried under reduced pressure to afford methyl N-[5-[(5-amino-2-chloro-4-pyridyl)amino]-2-pyridyl]carbamate.
1H NMR (400 MHz, DMSO-d6, ppm) δ = 10.12 (s, 1 H), 8.13 (s, 1 H), 7.52 - 8.85 (m, 4 H), 6.55 (s, 1 H), 5.00 (br. s, 2 H), 3.70 (s, 3 H) Step 3: Preparation of methyl N-[5-(6-chloroimidazo[4,5-c]pyridin-1-yl)-2-pyridyl]carbamate
A solution of methyl N-[5-[(5-amino-2-chloro-4-pyridyl)amino]-2-pyridyl]carbamate (2.00 g, 6.47 mmol) in diethoxymethoxyethane (10.0 mL) was charged with hydrogen chloride (36.0 %, 0.655 g, 6.47 mmol, 1.00 eq.) and stirred at 100 °C for 1 hour. The reaction mixture was concentrated under reduced pressure. The crude residue was diluted with water and stirred for 20 min. The solid was filtered off and washed with water, then washed with n-hexane and dried to afford methyl N-[5-(6-chloroimidazo[4,5- c]pyridin-1-yl)-2-pyridyl]carbamate.
1H NMR (400 MHz, DMSO-d6, ppm) δ = 10.63 (s, 1 H), 8.90 (s, 1 H), 8.77 (s, 1 H), 8.61 (s, 1 H), 8.00 - 8.21 (m, 2 H), 7.78 (s, 1 H), 3.72 (s, 3 H) Step 4: Preparation of methyl 1-[6-(methoxycarbonylamino)-3-pyridyl]imidazo[4,5-c]pyridine-6- carboxylate
A mixture of methyl N-[5-(6-chloroimidazo[4,5-c]pyridin-1-yl)-2-pyridyl]carbamate (3.00 g, 9.38 mmol) and sodium acetate (2.31 g, 28.2 mmol, 3.00 eq.) in methanol (100 mL) was degassed with argon for 10 min. Diphenyl-[rac-(4S,4'R)-1'-diphenylphosphanylferrocen-1-yl]phosphane (0.260 g, 0.469 mmol, 0.050 eq.) was added, followed by palladium diacetate (0.105 g, 0.469 mmol, 0.050 eq.). The reactor was pressurized with CO gas at 200 psi pressure and the reaction mixture was stirred at 100 °C for 16 hours. The reaction mixture was filtered, washing methanol, and the solid was dried to afford methyl 1- [6-(methoxycarbonylamino)-3-pyridyl]imidazo[4,5-c]pyridine-6-carboxylate.
1H NMR (400 MHz, DMSO-d6, ppm) δ = 10.60 (s, 1 H), 9.18 (s, 1 H), 8.89 (s, 1 H), 8.65 (s, 1 H), 8.00 – 8.30 (m, 3 H), 3.90 (s, 3 H), 3.73 (s, 3 H) Step 5: Preparation of [1-[6-(methoxycarbonylamino)-3-pyridyl]imidazo[4,5-c]pyridine-6- carbonyl]oxylithium
Lithium hydroxide hydrate (369 mg, 8.80 mmol, 4.00 equiv) was added to a solution of methyl 1-[6- (methoxycarbonylamino)-3-pyridyl]imidazo[4,5-c]pyridine-6-carboxylate (800 mg, 2.20 mmol) in THF (15.0 mL)/methanol (10.0 mL)/H2O (10.0 mL). The resulting reaction mixture was stirred at room temperature for 16 hours. The reaction mixture was concentrate under reduced pressure and the crude residue was diluted with water. The precipitate was filtered off and washed with methanol, then dried to afford [1-[6-(methoxycarbonylamino)-3-pyridyl]imidazo[4,5-c]pyridine-6-carbonyl]oxylithium. LC/MS (Method D) retention time = 2.02 min; [M+H-Li]
+ = 314.1 Step 6: Preparation of methyl N-[5-[6-[(4-fluorophenyl)-methyl-carbamoyl]imidazo[4,5-c]156yridine-1- yl]-2-pyridyl]carbamate (Compound 32) A solution of [1-[6-(methoxycarbonylamino)-3-pyridyl]imidazo[4,5-c]pyridine-6-carbonyl]oxylithium (200 mg, 0.595 mmol) and 4-fluoro-N-methyl-aniline (112 mg, 0.893 mmol, 1.50 eq.) in dimethylformamide (10.0 mL) was treated with diisopropylethylamine (0.321 mL, 1.79 mmol, 3.00 eq.) and 1- [bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (452 mg, 1.19 mmol, 2.00 eq.). The reaction was stirred at room temperature for 16 hours. The reaction was quenched by addition of water and the aqueous layer was concentrated under reduced pressure. The crude residue was purified by reverse phase chromatography (H2O/ acetonitrile) to afford methyl N-[5- [6-[(4-fluorophenyl)-methyl-carbamoyl]imidazo[4,5-c]pyridin-1-yl]-2-pyridyl]carbamate as an off white solid. LC/MS (Method D) retention time = 3.16 min; [M+H]
+ = 421.0
1H NMR (400 MHz, DMSO-d6, ppm) δ = 10.60 (s, 1 H), 8.78 (s, 2 H), 8.59 (m, 1 H), 8.02 – 8.17 (m, 2 H), 7.82 (s, 1 H), 7.15 – 7.28 (br. s, 2 H), 6.98 – 7.10 (br s, 2 H), 3.75 (s, 3 H), 3.39 (s, 3 H) Example 20: Preparation of methyl N-[5-[6-[(4-chlorophenyl)-methyl-carbamothioyl]-4-methyl- benzimidazol-1-yl]-2-pyridyl]carbamate (Compound 98)
(Compound 98) Step 1: Preparation of methyl N-[5-(6-bromo-4-methyl-benzimidazol-1-yl)-2-pyridyl]carbamate

Boric acid (0.200 g, 3.00 mmol, 2.00 eq.) and copper(II) acetate (0.400 g, 2.00 mmol, 1.5 eq.) were added to a solution of 6-bromo-4-methyl-1H-benzimidazole (0.300 g, 1.00 mmol) in acetonitrile (10 mL). Then, N,N-diisopropylethylamine (0.500 mL, 2.00 mmol, 2.00 eq.) was added followed by methyl N-[5- (4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2-pyridyl]carbamate (0.800 g, 3.00 mmol, 2.00 eq.). The reaction was stirred at 65 °C for 12 hours. The reaction mixture was poured into water. The aqueous layer was extracted with ethyl acetate, the combined organic layers were washed with water then brine, dried over sodium sulfate, filtered and concentrated under reduced pressure. The crude residue was purified by flash chromatography on silica gel (cyclohexane/ethylacetate) to afford methyl N-[5-(6- bromo-4-methyl-benzimidazol-1-yl)-2-pyridyl]carbamate as a pink solid. LC/MS (Method B) retention time = 1.07 min; [M+H]
+ = 361
1H NMR (400 MHz, DMSO-d6, ppm) δ = 2.95 (s, 3 H), 3.73 (s, 3 H), 7.32 (s, 1 H), 7.56 (d, J = 1.25 Hz, 1 H), 8.06 (d, J = 8.88 Hz, 1 H), 8.14 (dd, J = 8.88, 2.63 Hz, 1 H), 8.53 (s, 1 H), 8.58 (d, J = 2.13 Hz, 1 H), 10.57 (s, 1 H) Step 2: Preparation of methyl N-[5-[6-[(4-chlorophenyl)-methyl-carbamoyl]-4-methyl-benzimidazol-1-yl]- 2-pyridyl]carbamate (Compound 95)
(Compound 95) Bis(benzonitrile)palladium chloride (27.6 mg, 0.0277 mmol, 0.1 eq.), 4,5-bis(diphenylphosphino)-9,9- dimethylxanthene (16.3 mg, 0.0277 mmol, 0.10 eq.) and triethylamine (0.058 mL, 0.415 mmol, 1.50 eq.) were added to a solution of methyl N-[5-(6-bromo-4-methyl-benzimidazol-1-yl)-2-pyridyl]carbamate (100 mg, 0.277 mmol) and 4-chloro-N-methyl-aniline (0.277 mmol, 1.01 eq.) in toluene (1.0 mL), The vial was flushed with nitrogen then CO gas and then filled with 10 bar CO gas. The reaction was heated at 100 °C for 6 hours. The reaction mixture was filtered on celite using methanol, and the filtrate was concentrated under reduced pressure. The crude residue was purified by column chromatography on silica gel (cyclohexane/ethylacetate) to afford methyl N-[5-[6-[(4-chlorophenyl)-methyl-carbamoyl]-4- methyl-benzimidazol-1-yl]-2-pyridyl]carbamate as a white solid. LC/MS (Method B) retention time = 1.04 min; [M+H]
+ = 450
1H NMR (400 MHz, DMSO-d6, ppm) δ = 2.50 (m, 3 H), 3.35 (s, 3 H), 3.74 (s, 3 H), 7.16 (s, 2 H) 7.24 (m, 2 H), 7.34 (m, 2 H), 7.75 (dd, J = 8.88, 2.75 Hz, 1 H), 8.05 (d, J = 9.38 Hz, 1 H), 8.42 (d, J = 2.25 Hz, 1 H), 8.54 (s, 1 H) 10.59 (s, 1 H). Step 3: Preparation of methyl N-[5-[6-[(4-chlorophenyl)-methyl-carbamothioyl]-4-methyl-benzimidazol- 1-yl]-2-pyridyl]carbamate (Compound 98) A suspension of methyl N-[4-[6-[(4-fluoro-3-methoxy-phenyl)-methyl-carbamoyl]-4-methyl- benzimidazol-1-yl]phenyl]carbamate (25.0 mg, 0.0556 mmol) and Lawesson's reagent (CAS 19172-47- 5, 0.0162 g, 0.70 eq.) in toluene (0.83 mL) was heated to 110 °C for 2.5 hours. The reaction mixture was poured into a mixture of saturated aqueous sodium bicarbonate and ethyl acetate. The aqueous layer was extracted with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate, and concentrated under vacuum. The residue was purified by silica gel column chromatography (cyclohexane/ethyl acetate) to afford methyl N-[5-[6-[(4-chlorophenyl)-methyl- carbamothioyl]-4-methyl-benzimidazol-1-yl]-2-pyridyl]carbamate (Compound 98) as a light yellow solid. LC/MS (Method B) retention time = 1.14 min; [M+H]
+ = 466
1H NMR (400 MHz, DMSO-d6, ppm) δ = 2.51 (m, 3 H), 3.74 (br s, 3 H), 3.82 (br s, 3 H), 7.10(s, 1 H), 7.19(s, 1 H), 7.32 (br s, 4 H), 7.83 (s, 1 H), 8.07 (br d, J = 8.68 Hz, 1 H), 8.41 (br s, 1 H), 8.50 (br s, 1 H), 10.60 (br s, 1 H). Biological examples: The fungicidal activity of the compounds of the invention have been tested as follows: Phytophthora infestans / tomato / leaf disc preventative (late blight)
Tomato leaf disks are placed on water agar in multiwell plates (24-well format) and sprayed with the formulated test compound diluted in water. The leaf disks are inoculated with a spore suspension of the fungus 1 day after application. The inoculated leaf disks are incubated at 16 °C and 75% rh under a light regime of 24 h darkness followed by 12 h light / 12 h darkness in a climate cabinet and the activity of a compound is assessed as percent disease control compared to untreated when an appropriate level of disease damage appears in untreated check leaf disks (5 - 7 days after application). The following compounds gave at least 80% control of Phytophthora infestans at 200 ppm when compared to untreated control under the same conditions, which showed extensive disease development: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 71, 72, 94, 96, 97 Plasmopara viticola / grape / leaf disc preventative (late blight) Grape vine leaf disks are placed on water agar in multiwell plates (24-well format) and sprayed with the formulated test compound diluted in water. The leaf disks are inoculated with a spore suspension of the fungus 1 day after application. The inoculated leaf disks are incubated at 19 °C and 80% rh under a light regime of 12 h light / 12 h darkness in a climate cabinet and the activity of a compound is assessed as percent disease control compared to untreated when an appropriate level of disease damage appears in untreated check leaf disks (6 - 8 days after application). The following compounds gave at least 80% control of Plasmopara viticola at 200 ppm when compared to untreated control under the same conditions, which showed extensive disease development: 1, 3, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 16, 17, 18, 19, 20, 21, 22, 23, 25, 26, 27, 28, 29, 33, 36, 40, 55, 94, 96, 97 Pythium ultimum / liquid culture (seedling damping off) Mycelia fragments and oospores of a newly grown liquid culture of the fungus are directly mixed into nutrient broth (PDB potato dextrose broth). After placing a (DMSO) solution of test compound into a microtiter plate (96-well format), the nutrient broth containing the fungal mycelia/spore mixture is added. The test plates are incubated at 24 °C and the inhibition of growth is determined photometrically 2-3 days after application. The following compounds gave at least 80% control of Pythium ultimum at 20 ppm when compared to untreated control under the same conditions, which showed extensive disease development: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 34, 38, 39, 40, 42, 44, 45, 54, 55, 61, 65, 66, 72, 78, 79, 82, 88, 93, 94, 95, 96, 97