TRYPTAMINE DERIVATIVES Cross‐Reference to Related Applications [001] This application claims priority to U.S. Provisional Application No. 63/385,261, filed on November 29, 2022; U.S. Provisional Application No. 63/385,287, filed on November 29, 2022; U.S. Provisional Application No. 63/385,448, filed on November 30, 2022; U.S. Provisional Application No. 63/385,662, filed on December 1, 2022; U.S. Provisional Application No. 63/385,664, filed on December 1, 2022; U.S. Provisional Application No. 63/385,665, filed on December 1, 2022; U.S. Provisional Application No. 63/385,666, filed on December 1, 2022; U.S. Provisional Application No. 63/385,667, filed on December 1, 2022; U.S. Provisional Application No. 63/386,501, filed on December 8, 2022; U.S. Provisional Application No. 63/386,523, filed on December 8, 2022; U.S. Provisional Application No. 63/386,534, filed on December 8, 2022; U.S. Provisional Application No. 63/386,502, filed on December 8, 2022; and U.S. Provisional Application No. 63/386,503, filed on December 8, 2022; the disclosures of which are each incorporated herein by reference. Technical Field [002] This disclosure relates to bis(2‐(5‐methoxy‐1H‐indol‐3‐yl)ethan‐1‐aminium) (2E)‐but‐2‐ enedioate (5‐methoxytryptammonium fumarate or 5‐MeO‐T fumarate), crystalline 5‐MeO‐T fumarate, and specific crystalline forms thereof, including crystalline form 1 of 5‐MeO‐T fumarate; to pharmaceutical compositions containing 5‐MeO‐T fumarate or crystalline 5‐MeO‐T fumarate, including crystalline form 1 of 5‐MeO‐T fumarate; and to methods of treatment/therapeutic uses of 5‐MeO‐T fumarate or crystalline 5‐MeO‐T fumarate, including crystalline form 1 of 5‐MeO‐T fumarate. [003] This disclosure further relates to 2‐(1H‐indol‐3‐yl)ethan‐1‐aminium 3‐carboxy‐2‐ hydroxypropanoate (tryptammonium hydromalate), crystalline tryptammonium hydromalate, and specific crystalline forms thereof, including crystalline form 1 of tryptammonium hydromalate; to pharmaceutical compositions containing tryptammonium hydromalate or crystalline tryptammonium hydromalate, including crystalline form 1 of tryptammonium hydromalate; and to methods of treatment/therapeutic uses of tryptammonium hydromalate or crystalline tryptammonium hydromalate, including crystalline form 1 of tryptammonium hydromalate. [004] This disclosure further relates to 2‐(1H‐indol‐3‐yl)ethan‐1‐aminium 2‐carboxybenzoate (tryptammonium hydrophthalate), crystalline tryptammonium hydrophthalate, and specific crystalline forms thereof, including crystalline form 1 of tryptammonium hydrophthalate; to pharmaceutical compositions containing tryptammonium hydrophthalate or crystalline tryptammonium hydrophthalate, including crystalline form 1 of tryptammonium hydrophthalate;
and to methods of treatment/therapeutic uses of tryptammonium hydrophthalate or crystalline tryptammonium hydrophthalate, including crystalline form 1 of tryptammonium hydrophthalate. [005] This disclosure further relates to bis([2‐(4‐methoxy‐1H‐indol‐3‐yl)ethyl](methyl)(propan‐2‐ yl)azanium) (2E)‐but‐2‐enedioate (4‐methoxy‐N‐methyl‐N‐isopropyltryptammonium fumarate or 4‐ MeO‐MiPT fumarate), crystalline 4‐MeO‐MiPT fumarate, and specific crystalline forms thereof, including crystalline form 1 of 4‐MeO‐MiPT fumarate; to pharmaceutical compositions containing 4‐ MeO‐MiPT fumarate or crystalline 4‐MeO‐MiPT fumarate, including crystalline form 1 of 4‐MeO‐ MiPT fumarate; and to methods of treatment/therapeutic uses of 4‐MeO‐MiPT fumarate or crystalline 4‐MeO‐MiPT fumarate, including crystalline form 1 of 4‐MeO‐MiPT fumarate. [006] This disclosure further relates to [2‐(4‐hydroxy‐1H‐indol‐3‐yl)ethyl](propan‐2‐ yl)propylazanium (2E)‐3‐carboxyprop‐2‐enoate (4‐hydroxy‐N‐propyl‐N‐isopropyltryptammnonium hydrofumarate or 4‐HO‐PiPT hydrofumarate), crystalline 4‐HO‐PiPT hydrofumarate, and specific crystalline forms thereof, including crystalline form 1 of 4‐HO‐PiPT hydrofumarate; to pharmaceutical compositions containing 4‐HO‐PiPT hydrofumarate or crystalline 4‐HO‐PiPT hydrofumarate, including crystalline form 1 of 4‐HO‐PiPT hydrofumarate; and to methods of treatment/therapeutic uses of 4‐HO‐PiPT hydrofumarate or crystalline 4‐HO‐PiPT hydrofumarate, including crystalline form 1 of 4‐HO‐PiPT hydrofumarate. [007] This disclosure further relates to 2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium 2‐{[2‐(5‐chloro‐ 1H‐indol‐3‐yl)ethyl]azaniumyl}butanedioate (5‐chlorotryptammonium N‐succinyl‐5‐ chlorotryptammonium or 5‐Cl‐T∙N‐Suc‐5‐Cl‐T), crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, and specific crystalline forms thereof, including crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T; to pharmaceutical compositions containing 5‐Cl‐T∙N‐Suc‐5‐Cl‐T or crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, including crystalline form 1 of 5‐Cl‐ T∙N‐Suc‐5‐Cl‐T; and to methods of treatment/therapeutic uses of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T or crystalline 5‐ Cl‐T∙N‐Suc‐5‐Cl‐T, including crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T. [008] This disclosure further relates to 2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium 3‐carboxy‐2‐ hydroxypropanoate (5‐chlorotryptammonium hydromaleate or 5‐Cl‐T hydromaleate), crystalline 5‐ Cl‐T hydromaleate, and specific crystalline forms thereof, including crystalline form 1 of 5‐Cl‐T hydromaleate; to pharmaceutical compositions containing 5‐Cl‐T hydromaleate or crystalline 5‐Cl‐T hydromaleate, including crystalline form 1 of 5‐Cl‐T hydromaleate; and to methods of treatment/therapeutic uses of 5‐Cl‐T hydromaleate or crystalline 5‐Cl‐T hydromaleate, including crystalline form 1 of 5‐Cl‐T hydromaleate. [009] This disclosure further relates to 2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium 2‐ carboxybenzoate (5‐chlorotryptammonium hydrophthalate or 5‐Cl‐T hydrophthalate), crystalline 5‐ Cl‐T hydrophthalate, and specific crystalline forms thereof, including crystalline form 1 of 5‐Cl‐T
hydrophthalate; to pharmaceutical compositions containing 5‐Cl‐T hydrophthalate or crystalline 5‐ Cl‐T hydrophthalate, including crystalline form 1 of 5‐Cl‐T hydrophthalate; and to methods of treatment/therapeutic uses of 5‐Cl‐T hydrophthalate or crystalline 5‐Cl‐T hydrophthalate, including crystalline form 1 of 5‐Cl‐T hydrophthalate. [010] This disclosure further relates to bis(2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium) 2‐ hydroxybutanedioate (5‐chlorotryptammonium malate or 5‐Cl‐T malate), crystalline 5‐Cl‐T malate, and specific crystalline forms thereof, including crystalline form 1 of 5‐Cl‐T malate; to pharmaceutical compositions containing 5‐Cl‐T malate or crystalline 5‐Cl‐T malate, including crystalline form 1 of 5‐Cl‐T malate; and to methods of treatment/therapeutic uses of 5‐Cl‐T malate or crystalline 5‐Cl‐T malate, including crystalline form 1 of 5‐Cl‐T malate. [011] This disclosure further relates to 2‐(5‐methoxy‐1H‐indol‐3‐yl)ethan‐1‐aminium 2‐{[2‐(5‐ methoxy‐1H‐indol‐3‐yl)ethyl]azaniumyl}butanedioate (5‐methoxytryptammonium N‐succinyl‐5‐ methoxytryptammonium or 5‐MeO‐T∙N‐Suc‐5‐MeO‐T), crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, and specific crystalline forms thereof, including crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T; to pharmaceutical compositions containing 5‐MeO‐T∙N‐Suc‐5‐MeO‐T or crystalline 5‐MeO‐T∙N‐Suc‐5‐ MeO‐T, including crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T; and to methods of treatment/therapeutic uses of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T or crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, including crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T. [012] This disclosure further relates to 2‐(5‐methyl‐1H‐indol‐3‐yl)ethan‐1‐amine (5‐ methyltryptamine or 5‐Me‐T), crystalline 5‐Me‐T, and specific crystalline forms thereof, including crystalline form 1 of 5‐Me‐T; to pharmaceutical compositions containing 5‐Me‐T or crystalline 5‐Me‐ T, including crystalline form 1 of 5‐Me‐T; and to methods of treatment/therapeutic uses of 5‐Me‐T or crystalline 5‐Me‐T, including crystalline form 1 of 5‐Me‐T. [013] This disclosure further relates to bis(N‐[2‐(4‐hydroxy‐1H‐indol‐3‐yl)ethyl]‐N‐ methylcyclopropanaminium) (2E)‐but‐2‐enedioate (4‐hydroxy‐N‐methyl‐N‐ cyclopropyltryptammonium fumarate or 4‐HO‐MCPT fumarate), crystalline 4‐HO‐MCPT fumarate, and specific crystalline forms thereof, including crystalline form 1 of 4‐HO‐MCPT fumarate; to pharmaceutical compositions containing 4‐HO‐MCPT fumarate or crystalline 4‐HO‐MCPT fumarate, including crystalline form 1 of 4‐HO‐MCPT fumarate; and to methods of treatment/therapeutic uses of 4‐HO‐MCPT fumarate or crystalline 4‐HO‐MCPT fumarate, including crystalline form 1 of 4‐HO‐ MCPT fumarate. [014] This disclosure further relates to [2‐(4‐hydroxy‐1H‐indol‐3‐yl)ethyl]bis(propan‐2‐yl)azanium (2E)‐3‐carboxyprop‐2‐enoate (4‐hydroxy‐N,N‐diisopropyltryptammonium hydrofumarate or 4‐HO‐ DiPT hydrofumarate), crystalline 4‐HO‐DiPT hydrofumarate, and specific crystalline forms thereof,
including crystalline form 1 of 4‐HO‐DiPT hydrofumarate; to pharmaceutical compositions containing 4‐HO‐DiPT hydrofumarate or crystalline 4‐HO‐DiPT hydrofumarate, including crystalline form 1 of 4‐ HO‐DiPT hydrofumarate; and to methods of treatment/therapeutic uses of 4‐HO‐DiPT hydrofumarate or crystalline 4‐HO‐DiPT hydrofumarate, including crystalline form 1 of 4‐HO‐DiPT hydrofumarate. Background of the Invention [015] Obtaining specific salts or crystalline forms of an active pharmaceutical ingredient (API) is extremely useful in drug development. It permits better characterization of the drug candidate’s chemical and physical properties. Crystalline forms often have better chemical and physical properties than the API in its amorphous state. Such crystalline forms may possess more favorable pharmaceutical and pharmacological properties or be easier to process. Additionally, preparing a crystalline API and solving its crystal structure provides the gold standard for chemical characterization and determining the molecular formula (and molecular weight) of the API. Accordingly, preparing a crystalline form with an accompanying crystal structure thereof prevents potential ambiguities and/or inaccuracies in the API’s molecular weight. This is important because the API’s molecular weight is used to calculate the concentration of compositions comprising that API. Thus, inaccuracies in molecular weight may lead to errors in the calculations pertaining to dosing, potency, toxicity, etc. in all downstream in vitro and in vivo assays that correlated the concentration of the API with a measured property. Accordingly, there remains a need to obtain and characterize crystalline forms of APIs, such as tryptamines and other psychedelic drug compounds. Summary of the Invention [016] This disclosure relates to bis(2‐(5‐methoxy‐1H‐indol‐3‐yl)ethan‐1‐aminium) (2E)‐but‐2‐ enedioate (5‐methoxytryptammonium fumarate or 5‐MeO‐T fumarate), crystalline 5‐MeO‐T fumarate, and specific crystalline forms thereof. In one embodiment, this disclosure pertains to particular crystalline forms of 5‐MeO‐T fumarate, including crystalline form 1 of 5‐MeO‐T fumarate. In one embodiment, crystalline form 1 of 5‐MeO‐T fumarate is characterized by at least one of: a monoclinic, P2
1/c space group at a temperature of about 297(2) K; unit cell dimensions a = 5.8070(3) Å, b = 24.2768(13) Å, c = 8.9334(5) Å, α = 90°, β = 103.360(2)°, and ɣ = 90°; an X‐ray powder diffraction (XRPD) pattern substantially similar to FIG. 30; and an X‐ray powder diffraction pattern characterized by at least two peaks selected from 7.3, 12.5, and 24.8 °2θ ± 0.2 °2θ. [017] This disclosure further relates to 2‐(1H‐indol‐3‐yl)ethan‐1‐aminium 3‐carboxy‐2‐ hydroxypropanoate (tryptammonium hydromalate), crystalline tryptammonium hydromalate, and specific crystalline forms thereof. In one embodiment, this disclosure pertains to particular crystalline forms of tryptammonium hydromalate, including crystalline form 1 of tryptammonium
hydromalate. In one embodiment, crystalline form 1 of tryptammonium hydromalate is characterized by at least one of: an orthorhombic, P2
12
12
1 space group at a temperature of about 297(2) K; unit cell dimensions a = 7.2012(5) Å, b = 8.2509(5) Å, c = 24.7541(16) Å, α = 90°, β = 90°, and ɣ = 90°; an X‐ray powder diffraction (XRPD) pattern substantially similar to FIG. 31; and an X‐ray powder diffraction pattern characterized by at least two peaks selected from 7.1, 11.3, and 20.9 °2θ ± 0.2 °2θ. [018] This disclosure further relates to 2‐(1H‐indol‐3‐yl)ethan‐1‐aminium 2‐carboxybenzoate (tryptammonium hydrophthalate), crystalline tryptammonium hydrophthalate, and specific crystalline forms thereof. In one embodiment, this disclosure pertains to particular crystalline forms of tryptammonium hydrophthalate, including crystalline form 1 of tryptammonium hydrophthalate. In one embodiment, crystalline form 1 of tryptammonium hydrophthalate is characterized by at least one of: a monoclinic, C2/c space group at a temperature of about 297(2) K; unit cell dimensions a = 18.7823(11) Å, b = 5.7731(3) Å, c = 29.3825(19) Å, α = 90°, β = 90.617(2)°, and ɣ = 90°; an X‐ray powder diffraction (XRPD) pattern substantially similar to FIG. 32; and an X‐ray powder diffraction pattern characterized by at least two peaks selected from 6.0, 11.2, and 15.4 °2θ ± 0.2 °2θ. [019] This disclosure further relates to bis([2‐(4‐methoxy‐1H‐indol‐3‐yl)ethyl](methyl)(propan‐2‐ yl)azanium) (2E)‐but‐2‐enedioate (4‐methoxy‐N‐methyl‐N‐isopropyltryptammonium fumarate or 4‐ MeO‐MiPT fumarate), crystalline 4‐MeO‐MiPT fumarate, and specific crystalline forms thereof. In one embodiment, this disclosure pertains to particular crystalline forms of 4‐MeO‐MiPT fumarate, including crystalline form 1 of 4‐MeO‐MiPT fumarate. In one embodiment, crystalline form 1 of 4‐ MeO‐MiPT fumarate is characterized by at least one of: a monoclinic, P2
1/n space group at a temperature of about 297(2) K; unit cell dimensions a = 9.1438(3) Å, b = 13.8957(6) Å, c = 13.6658(6) Å, α = 90°, β = 94.6140(10)°, and ɣ = 90°; an X‐ray powder diffraction (XRPD) pattern substantially similar to FIG. 33; and an X‐ray powder diffraction pattern characterized by at least two peaks selected from 9.1, 14.5, and 16.0 °2θ ± 0.2 °2θ. [020] This disclosure further relates to [2‐(4‐hydroxy‐1H‐indol‐3‐yl)ethyl](propan‐2‐ yl)propylazanium (2E)‐3‐carboxyprop‐2‐enoate (4‐hydroxy‐N‐propyl‐N‐isopropyltryptammnonium hydrofumarate or 4‐HO‐PiPT hydrofumarate), crystalline 4‐HO‐PiPT hydrofumarate, and specific crystalline forms thereof. In one embodiment, this disclosure pertains to particular crystalline forms of 4‐HO‐PiPT hydrofumarate, including crystalline form 1 of 4‐HO‐PiPT hydrofumarate. In one embodiment, crystalline form 1 of 4‐HO‐PiPT hydrofumarate is characterized by at least one of: an orthorhombic, P2
12
12
1 space group at a temperature of about 297(2) K; unit cell dimensions a = 7.8854(7) Å, b = 12.7401(11) Å, c = 20.5577(18) Å, α = 90°, β = 90°, and ɣ = 90°; an X‐ray powder
diffraction (XRPD) pattern substantially similar to FIG. 34; and an X‐ray powder diffraction pattern characterized by at least two peaks selected from 11.1, 12.0, and 15.8 °2θ ± 0.2 °2θ. [021] This disclosure further relates to 2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium 2‐{[2‐(5‐chloro‐ 1H‐indol‐3‐yl)ethyl]azaniumyl}butanedioate (5‐chlorotryptammonium N‐succinyl‐5‐ chlorotryptammonium or 5‐Cl‐T∙N‐Suc‐5‐Cl‐T), crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, and specific crystalline forms thereof. In one embodiment, this disclosure pertains to particular crystalline forms of 5‐Cl‐ T∙N‐Suc‐5‐Cl‐T, including crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T. In one embodiment, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T is characterized by at least one of: a triclinic, P1
ത space group at a temperature of about 300(2) K; unit cell dimensions a = 9.5806(13) Å, b = 11.1666(14) Å, c = 11.5518(17) Å, α = 87.333(5)°, β = 74.347(5)°, and ɣ = 85.637(5)°; an X‐ray powder diffraction (XRPD) pattern substantially similar to FIG. 35; and an X‐ray powder diffraction pattern characterized by at least two peaks selected from 12.8, 15.9, and 22.9 °2θ ± 0.2 °2θ. [022] This disclosure further relates to 2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium 3‐carboxy‐2‐ hydroxypropanoate (5‐chlorotryptammonium hydromaleate or 5‐Cl‐T hydromaleate), crystalline 5‐ Cl‐T hydromaleate, and specific crystalline forms thereof. In one embodiment, this disclosure pertains to particular crystalline forms of 5‐Cl‐T hydromaleate, including crystalline form 1 of 5‐Cl‐T hydromaleate. In one embodiment, crystalline form 1 of 5‐Cl‐T hydromaleate is characterized by at least one of: a monoclinic, P2
1/c space group at a temperature of about 300(2) K; unit cell dimensions a = 9.6320(7) Å, b = 5.4224(4) Å, c = 28.494(2) Å, α = 90°, β = 95.758(3)°, and ɣ = 90°; an X‐ray powder diffraction (XRPD) pattern substantially similar to FIG. 36; and an X‐ray powder diffraction pattern characterized by at least two peaks selected from 9.2, 10.6, and 14.8 °2θ ± 0.2 °2θ. [023] This disclosure further relates to 2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium 2‐ carboxybenzoate (5‐chlorotryptammonium hydrophthalate or 5‐Cl‐T hydrophthalate), crystalline 5‐ Cl‐T hydrophthalate, and specific crystalline forms thereof. In one embodiment, this disclosure pertains to particular crystalline forms of 5‐Cl‐T hydrophthalate, including crystalline form 1 of 5‐Cl‐T hydrophthalate. In one embodiment, crystalline form 1 of 5‐Cl‐T hydrophthalate is characterized by at least one of: a triclinic, P1
ത space group at a temperature of about 300(2) K; unit cell dimensions a = 7.4013(6) Å, b = 9.8217(9) Å, c = 12.1614(12) Å, α = 83.285(3)°, β = 79.601(3)°, and ɣ = 78.437(3)°; an X‐ray powder diffraction (XRPD) pattern substantially similar to FIG. 37; and an X‐ray powder diffraction pattern characterized by at least two peaks selected from 11.3, 12.3, and 15.4 °2θ ± 0.2 °2θ. [024] This disclosure further relates to bis(2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium) 2‐ hydroxybutanedioate (5‐chlorotryptammonium malate or 5‐Cl‐T malate), crystalline 5‐Cl‐T malate,
and specific crystalline forms thereof. In one embodiment, this disclosure pertains to particular crystalline forms of 5‐Cl‐T malate, including crystalline form 1 of 5‐Cl‐T malate. In one embodiment, crystalline form 1 of 5‐Cl‐T malate is characterized by at least one of: a monoclinic, P2
1 space group at a temperature of about 300(2) K; unit cell dimensions a = 8.9975(8) Å, b = 12.3798(12) Å, c = 11.3628(11) Å, α = 90°, β = 106.617(3)°, and ɣ = 90°; an X‐ray powder diffraction (XRPD) pattern substantially similar to FIG. 38; and an X‐ray powder diffraction pattern characterized by at least two peaks selected from 12.5, 13.2, and 14.3 °2θ ± 0.2 °2θ. [025] This disclosure further relates to 2‐(5‐methoxy‐1H‐indol‐3‐yl)ethan‐1‐aminium 2‐{[2‐(5‐ methoxy‐1H‐indol‐3‐yl)ethyl]azaniumyl}butanedioate (5‐methoxytryptammonium N‐succinyl‐5‐ methoxytryptammonium or 5‐MeO‐T∙N‐Suc‐5‐MeO‐T), crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, and specific crystalline forms thereof. In one embodiment, this disclosure pertains to particular crystalline forms of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, including crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐ T. In one embodiment, crystalline form 5‐MeO‐T∙N‐Suc‐5‐MeO‐T is characterized by at least one of: a triclinic, P1
ത space group at a temperature of about 300(2) K; unit cell dimensions a = 9.5838(10) Å, b = 10.9915(12) Å, c = 12.6894(11) Å, α = 87.708(3)°, β = 71.246(3)°, and ɣ = 84.805(4)°; an X‐ray powder diffraction (XRPD) pattern substantially similar to FIG. 39; and an X‐ray powder diffraction pattern characterized by at least two peaks selected from 7.4, 18.4, and 19.6 °2θ ± 0.2 °2θ. [026] This disclosure further relates to 2‐(5‐methyl‐1H‐indol‐3‐yl)ethan‐1‐amine (5‐ methyltryptamine or 5‐Me‐T), crystalline 5‐Me‐T, and specific crystalline forms thereof. In one embodiment, this disclosure pertains to particular crystalline forms of 5‐Me‐T, including crystalline form 1 of 5‐Me‐T. In one embodiment, crystalline form 1 of 5‐Me‐T is characterized by at least one of: an orthorhombic, Pbca space group at a temperature of about 300(2) K; unit cell dimensions a = 9.1099(11) Å, b = 11.3907(11) Å, c = 19.154(2) Å, α = 90°, β = 90°, and ɣ = 90°; an X‐ray powder diffraction (XRPD) pattern substantially similar to FIG. 40; and an X‐ray powder diffraction pattern characterized by at least two peaks selected from 13.4, 15.5, and 19.5 °2θ ± 0.2 °2θ. [027] This disclosure further relates to bis(N‐[2‐(4‐hydroxy‐1H‐indol‐3‐yl)ethyl]‐N‐ methylcyclopropanaminium) (2E)‐but‐2‐enedioate (4‐hydroxy‐N‐methyl‐N‐ cyclopropyltryptammonium fumarate or 4‐HO‐MCPT fumarate), crystalline 4‐HO‐MCPT fumarate, and specific crystalline forms thereof. In one embodiment, this disclosure pertains to particular crystalline forms of 4‐HO‐MCPT fumarate, including crystalline form 1 of 4‐HO‐MCPT fumarate. In one embodiment, crystalline form 1 of 4‐HO‐MCPT fumarate is characterized by at least one of: a monoclinic, C2/c space group at a temperature of about 300(2) K; unit cell dimensions a = 19.5453(17) Å, b = 9.3265(7) Å, c = 17.4448(16) Å, α = 90°, β = 109.390(3)°, and ɣ = 90°; an X‐ray
powder diffraction (XRPD) pattern substantially similar to FIG. 41; and an X‐ray powder diffraction pattern characterized by at least two peaks selected from 9.6, 10.7, and 16.6 °2θ ± 0.2 °2θ. [028] This disclosure further relates to [2‐(4‐hydroxy‐1H‐indol‐3‐yl)ethyl]bis(propan‐2‐yl)azanium (2E)‐3‐carboxyprop‐2‐enoate (4‐hydroxy‐N,N‐diisopropyltryptammonium hydrofumarate or 4‐HO‐ DiPT hydrofumarate), crystalline 4‐HO‐DiPT hydrofumarate, and specific crystalline forms thereof. In one embodiment, this disclosure pertains to particular crystalline forms of 4‐HO‐DiPT hydrofumarate, including crystalline form 1 of 4‐HO‐DiPT hydrofumarate. In one embodiment, crystalline form 1 of 4‐HO‐DiPT hydrofumarate is characterized by at least one of: an orthorhombic, P2
12
12
1 space group at a temperature of about 300(2) K; unit cell dimensions a = 7.9541(3) Å, b = 12.5763(5) Å, c = 20.3351(6) Å, α = 90°, β = 90°, and ɣ = 90°; an X‐ray powder diffraction (XRPD) pattern substantially similar to FIG. 42; and an X‐ray powder diffraction pattern characterized by at least two peaks selected from 8.3, 11.2, and 15.8 °2θ ± 0.2 °2θ. [029] The disclosure further relates to a composition comprising 5‐MeO‐T fumarate, crystalline 5‐ MeO‐T fumarate, or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, and at least one excipient. [030] The disclosure further relates to a composition comprising tryptammonium hydromalate, crystalline tryptammonium hydromalate, or specific crystalline forms thereof, such as crystalline form 1 of tryptammonium hydromalate, and at least one excipient. [031] The disclosure further relates to a composition comprising tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, or specific crystalline forms thereof, such as crystalline form 1 of tryptammonium hydrophthalate, and at least one excipient. [032] The disclosure further relates to a composition comprising 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, or specific crystalline forms thereof, such as crystalline form 1 of 4‐MeO‐ MiPT fumarate, and at least one excipient. [033] The disclosure further relates to a composition comprising 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, or specific crystalline forms thereof, such as crystalline form 1 of 4‐HO‐PiPT hydrofumarate, and at least one excipient. [034] The disclosure further relates to a composition comprising 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐ Cl‐T∙N‐Suc‐5‐Cl‐T, or specific crystalline forms thereof, such as crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐ T, and at least one excipient. [035] The disclosure further relates to a composition comprising 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, or specific crystalline forms thereof, such as crystalline form 1 of 5‐Cl‐T hydromaleate, and at least one excipient.
[036] The disclosure further relates to a composition comprising 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, or specific crystalline forms thereof, such as crystalline form 1 of 5‐Cl‐T hydrophthalate, and at least one excipient. [037] The disclosure further relates to a composition comprising 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, or specific crystalline forms thereof, such as crystalline form 1 of 5‐Cl‐T malate, and at least one excipient. [038] The disclosure further relates to a composition comprising 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, and at least one excipient. [039] The disclosure further relates to a composition comprising 5‐Me‐T, crystalline 5‐Me‐T, or specific crystalline forms thereof, such as crystalline form 1 of 5‐Me‐T, and at least one excipient. [040] The disclosure further relates to a composition comprising 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, or specific crystalline forms thereof, such as crystalline form 1 of 4‐HO‐MCPT fumarate, and at least one excipient. [041] The disclosure further relates to a composition comprising 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate, or specific crystalline forms thereof, such as crystalline form 1 of 4‐HO‐DiPT hydrofumarate, and at least one excipient. [042] The disclosure also provides a composition comprising 5‐MeO‐T fumarate, crystalline 5‐ MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate, or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐ T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate, as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic
drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone; and at least one excipient. [043] The disclosure also relates to a method of preventing or treating a psychological disorder comprising the step of administering to a subject in need thereof a therapeutically effective amount of 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate, or a composition according to this disclosure. [044] The disclosure further relates to a method of preventing or treating inflammation and/or pain, preventing or treating a neurological disorder, modulating activity of a mitogen‐activated protein kinase (MAPK), modulating neurogenesis, or modulating neurite outgrowth comprising the step of administering to a subject in need thereof a therapeutically effective amount of a compound of 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐
Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate, and to administering a pharmaceutical composition or a composition according to the invention. [045] As used herein, the term “a subject in need thereof” refers to a person requiring a composition to treat a particular disease or condition (e.g., inflammation, pain, a psychological disorder, modulating activity at a receptor, etc.). In one embodiment, the “subject in need thereof” may be identified by analyzing, diagnosing, and/or determining whether the person (or subject) requires the composition for treatment of a particular disease or condition. In one embodiment, identifying a person in need of treatment comprises diagnosing a person with a medical condition, e.g., a neurological disorder, a chemical imbalance, a hereditary condition, etc. In one embodiment, identifying a person in need of treatment comprises performing a psychiatric evaluation. In one embodiment, identifying a person in need of treatment comprises performing a blood test. In one embodiment, identifying a person in need of treatment comprises determining whether a person has a compulsive disorder. In one embodiment, identifying a person in need of treatment comprises self‐identifying as having a compulsive disorder. Description of the Figures [046] FIG. 1 shows the molecular structure of crystalline form 1 of 5‐methoxytryptammonium fumarate. [047] FIG. 2 shows the molecular structure of crystalline form 1 of tryptammonium hydromalate. [048] FIG. 3 shows the molecular structure of crystalline form 1 of tryptammonium hydrophthalate. [049] FIG. 4 shows the molecular structure of crystalline form 1 of 4‐methoxy‐N‐methyl‐N‐ isopropyltryptammonium fumarate. [050] FIG. 5 shows the molecular structure of crystalline form 1 of 4‐hydroxy‐N‐propyl‐N‐ isopropyltryptammnonium hydrofumarate. [051] FIG. 6 shows the molecular structure of crystalline form 1 of 5‐chlorotryptammonium N‐ succinyl‐5‐chlorotryptammonium. [052] FIG. 7 shows the molecular structure of crystalline form 1 of 5‐chlorotryptammonium hydromaleate. [053] FIG. 8 shows the molecular structure of crystalline form 1 of 5‐chlorotryptammonium hydrophthalate.
[054] FIG. 9 shows the molecular structure of crystalline form 1 of 5‐chlorotryptammonium malate. [055] FIG. 10 shows the molecular structure of crystalline form 1 of 5‐methoxytryptammonium N‐ succinyl‐5‐methoxytryptammonium. [056] FIG. 11 shows the molecular structure of crystalline form 1 of 5‐methyltryptamine. [057] FIG. 12 shows the molecular structure of crystalline form 1 of 4‐hydroxy‐N‐methyl‐N‐ cyclopropyltryptammonium fumarate. [058] FIG. 13 shows the molecular structure of crystalline form 1 of 4‐hydroxy‐N,N‐ diisopropyltryptammonium hydrofumarate. [059] FIG. 14 shows the unit cell of crystalline form 1 of 5‐methoxytryptammonium fumarate along the a‐axis. [060] FIG. 15 shows the unit cell of crystalline form 1 of tryptammonium hydromalate along the a‐ axis. [061] FIG. 16 shows the unit cell of crystalline form 1 of tryptammonium hydrophthalate along the b‐axis. [062] FIG. 17 shows the unit cell of crystalline form 1 of 4‐methoxy‐N‐methyl‐N‐ isopropyltryptammonium fumarate along the c‐axis. [063] FIG. 18 shows the unit cell of crystalline form 1 of 4‐hydroxy‐N‐propyl‐N‐ isopropyltryptammnonium hydrofumarate along the a‐axis. [064] FIG. 19 shows the unit cell of crystalline form 1 of 5‐chlorotryptammonium N‐succinyl‐5‐ chlorotryptammonium along the a‐axis. [065] FIG. 20 shows the unit cell of crystalline form 1 of 5‐chlorotryptammonium hydromaleate along the b‐axis. [066] FIG. 21 shows the unit cell of crystalline form 1 of 5‐chlorotryptammonium hydrophthalate along the c‐axis. [067] FIG. 22 shows the unit cell of crystalline form 1 of 5‐chlorotryptammonium malate along the a‐axis. [068] FIG. 23 shows the unit cell of crystalline form 1 of 5‐methoxytryptammonium N‐succinyl‐5‐ methoxytryptammonium along the a‐axis. [069] FIG. 24 shows the unit cell of crystalline form 1 of 5‐methyltryptamine along the a‐axis. [070] FIG. 25 shows the unit cell of crystalline form 1 of 4‐hydroxy‐N‐methyl‐N‐ cyclopropyltryptammonium fumarate along the b‐axis. [071] FIG. 26 shows the unit cell of crystalline form 1 of 4‐hydroxy‐N,N‐diisopropyltryptammonium hydrofumarate along the a‐axis.
[072] FIG. 27 shows the 2:1 ratio of crystalline form 1 of 5‐methoxytryptammonium fumarate as a dimer. [073] FIG. 28 shows the 2:1 ratio of crystalline form 1 of 4‐methoxy‐N‐methyl‐N‐ isopropyltryptammonium fumarate as a dimer. [074] FIG. 29 shows the 2:1 ratio of crystalline form 1 of 4‐hydroxy‐N‐methyl‐N‐ cyclopropyltryptammonium fumarate as a dimer. [075] FIG. 30 shows the simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of 5‐methoxytryptammonium fumarate. [076] FIG. 31 shows the simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of tryptammonium hydromalate. [077] FIG. 32 shows the simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of tryptammonium hydrophthalate. [078] FIG. 33 shows the simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of 4‐methoxy‐N‐methyl‐N‐isopropyltryptammonium fumarate. [079] FIG. 34 shows the simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of 4‐hydroxy‐N‐propyl‐N‐isopropyltryptammnonium hydrofumarate. [080] FIG. 35 shows the simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of 5‐chlorotryptammonium N‐succinyl‐5‐chlorotryptammonium. [081] FIG. 36 shows the simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of 5‐chlorotryptammonium hydromaleate. [082] FIG. 37 shows the simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of 5‐chlorotryptammonium hydrophthalate. [083] FIG. 38 shows the simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of 5‐chlorotryptammonium malate. [084] FIG. 39 shows the simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of 5‐methoxytryptammonium N‐succinyl‐5‐methoxytryptammonium. [085] FIG. 40 shows the simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of 5‐methyltryptamine. [086] FIG. 41 shows the simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of 4‐hydroxy‐N‐methyl‐N‐cyclopropypltrytammonium fumarate. [087] FIG. 42 shows the simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of 4‐hydroxy‐N,N‐diisopropyltryptammonium hydrofumarate. Detailed Description [088] Compounds
[089] This disclosure relates to bis(2‐(5‐methoxy‐1H‐indol‐3‐yl)ethan‐1‐aminium) (2E)‐but‐2‐ enedioate (5‐methoxytryptammonium fumarate or 5‐MeO‐T fumarate), crystalline 5‐MeO‐T fumarate, 2‐(1H‐indol‐3‐yl)ethan‐1‐aminium 3‐carboxy‐2‐hydroxypropanoate (tryptammonium hydromalate), crystalline tryptammonium hydromalate, 2‐(1H‐indol‐3‐yl)ethan‐1‐aminium 2‐ carboxybenzoate (tryptammonium hydrophthalate), crystalline tryptammonium hydrophthalate, bis([2‐(4‐methoxy‐1H‐indol‐3‐yl)ethyl](methyl)(propan‐2‐yl)azanium) (2E)‐but‐2‐enedioate (4‐ methoxy‐N‐methyl‐N‐isopropyltryptammonium fumarate or 4‐MeO‐MiPT fumarate), crystalline 4‐ MeO‐MiPT fumarate, [2‐(4‐hydroxy‐1H‐indol‐3‐yl)ethyl](propan‐2‐yl)propylazanium (2E)‐3‐ carboxyprop‐2‐enoate (4‐hydroxy‐N‐propyl‐N‐isopropyltryptammnonium hydrofumarate or 4‐HO‐ PiPT hydrofumarate), crystalline 4‐HO‐PiPT hydrofumarate, 2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐ aminium 2‐{[2‐(5‐chloro‐1H‐indol‐3‐yl)ethyl]azaniumyl}butanedioate (5‐chlorotryptammonium N‐ succinyl‐5‐chlorotryptammonium or 5‐Cl‐T∙N‐Suc‐5‐Cl‐T), crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 2‐(5‐chloro‐ 1H‐indol‐3‐yl)ethan‐1‐aminium 3‐carboxy‐2‐hydroxypropanoate (5‐chlorotryptammonium hydromaleate or 5‐Cl‐T hydromaleate), crystalline 5‐Cl‐T hydromaleate, 2‐(5‐chloro‐1H‐indol‐3‐ yl)ethan‐1‐aminium 2‐carboxybenzoate (5‐chlorotryptammonium hydrophthalate or 5‐Cl‐T hydrophthalate), crystalline 5‐Cl‐T hydrophthalate, bis(2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium) 2‐hydroxybutanedioate (5‐chlorotryptammonium malate or 5‐Cl‐T malate), crystalline 5‐Cl‐T malate, 2‐(5‐methoxy‐1H‐indol‐3‐yl)ethan‐1‐aminium 2‐{[2‐(5‐methoxy‐1H‐indol‐3‐ yl)ethyl]azaniumyl}butanedioate (5‐methoxytryptammonium N‐succinyl‐5‐methoxytryptammonium or 5‐MeO‐T∙N‐Suc‐5‐MeO‐T), crystalline or 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 2‐(5‐methyl‐1H‐indol‐3‐ yl)ethan‐1‐amine (5‐methyltryptamine or 5‐Me‐T), crystalline 5‐Me‐T, bis(N‐[2‐(4‐hydroxy‐1H‐indol‐ 3‐yl)ethyl]‐N‐methylcyclopropanaminium) (2E)‐but‐2‐enedioate (4‐hydroxy‐N‐methyl‐N‐ cyclopropyltryptammonium fumarate or 4‐HO‐MCPT fumarate), crystalline 4‐HO‐MCPT fumarate, [2‐ (4‐hydroxy‐1H‐indol‐3‐yl)ethyl]bis(propan‐2‐yl)azanium (2E)‐3‐carboxyprop‐2‐enoate (4‐hydroxy‐ N,N‐diisopropyltryptammonium hydrofumarate or 4‐HO‐DiPT hydrofumarate), crystalline 4‐HO‐DiPT hydrofumarate, and specific crystalline forms thereof, including crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate; to pharmaceutical compositions containing 5‐ MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium
hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate according to the disclosure. The therapeutic uses of 5‐MeO‐T fumarate, crystalline 5‐ MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐ T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate according to the disclosure are described below as well as compositions containing them. 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐ MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T,
crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐ T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate, and some exemplary methods used to characterize them are described below. [090] 5‐MeO‐T fumarate has the following chemical formula:

[091] Tryptammonium hydromalate has the following chemical formula:
[092] Tryptammonium hydrophthalate has the following chemical formula:
[093] 4‐MeO‐MiPT fumarate has the following chemical formula:
[094] 4‐HO‐PiPT hydrofumarate has the following chemical formula:
[095] 5‐Cl‐T∙N‐Suc‐5‐Cl‐T has the following chemical formula:
[096] 5‐Cl‐T hydromaleate has the following chemical formula:
[097] 5‐Cl‐T hydrophthalate has the following chemical formula:
[098] 5‐Cl‐T malate has the following chemical formula:
[099] 5‐MeO‐T∙N‐Suc‐5‐MeO‐T has the following chemical formula:
[100] 5‐Me‐T has the following chemical formula:
[101] 4‐HO‐MCPT fumarate has the following chemical formula:
[102] 4‐HO‐DiPT hydrofumarate has the following chemical formula:
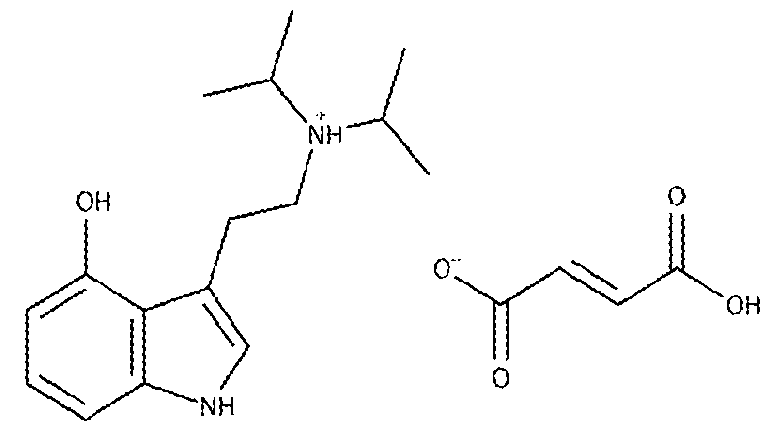
[103] Methods of Treatment and Therapeutic Uses [104] 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate according to the disclosure, and the methods and the compositions (e.g., pharmaceutical compositions) are used to regulate the activity of a neurotransmitter receptor by administering a therapeutically effective dose of 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐ MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐
5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐ T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure. In one embodiment, 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐ MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐ T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate according to the disclosure, and the methods and the compositions (e.g., pharmaceutical compositions) are used to treat inflammation and/or pain by administering a therapeutically effective dose of 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐ MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT
hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐ T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure. [105] Methods of the disclosure also relate to the administration of a therapeutically effective amount of 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐ PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐ Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐ Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate to prevent or treat a disease or condition, such as those discussed below for a subject in need of treatment. 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐ PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐ Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐ Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐
MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate may be administered neat or as a composition comprising 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐ MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐ T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate as discussed below. [106] 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT
hydrofumarate of the disclosure may be used to prevent and/or treat a psychological disorder. The disclosure provides a method for preventing and/or treating a psychological disorder by administering to a subject in need thereof a therapeutically effective amount of 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐ MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐ T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure, including the exemplary embodiments discussed herein. The psychological disorder may be chosen from: depression; psychotic disorder; schizophrenia; schizophreniform disorder (acute schizophrenic episode); schizoaffective disorder; bipolar I disorder (mania, manic disorder, manic‐ depressive psychosis); bipolar II disorder; major depressive disorder; major depressive disorder with psychotic feature (psychotic depression); delusional disorders (paranoia); shared psychotic disorder (shared paranoia disorder); brief psychotic disorder (other and unspecified reactive psychosis); psychotic disorder not otherwise specified (unspecified psychosis); paranoid personality disorder; schizoid personality disorder; schizotypal personality disorder; anxiety disorder; social anxiety disorder; substance‐induced anxiety disorder; selective mutism; panic disorder; panic attacks; agoraphobia; attention deficit syndrome; post‐traumatic stress disorder (PTSD); premenstrual dysphoric disorder (PMDD); and premenstrual syndrome (PMS). [107] 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐
MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure may be used to prevent and/or treat a brain disorder. The disclosure provides a method for preventing and/or treating a brain disorder (e.g., Huntington's disease, Alzheimer's disease, dementia, and Parkinson's disease) by administering to a subject in need thereof a therapeutically effective amount of 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐ MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐ 5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐ T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate. [108] 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline
forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure may be used to prevent and/or treat developmental disorders, delirium, dementia, amnestic disorders and other cognitive disorders, psychiatric disorders due to a somatic condition, drug‐related disorders, schizophrenia and other psychotic disorders, mood disorders, anxiety disorders, somatoform disorders, factitious disorders, dissociative disorders, eating disorders, sleep disorders, impulse control disorders, adjustment disorders, or personality disorders. The disclosure provides a method for preventing and/or treating these disorders by administering to a subject in need thereof a therapeutically effective amount of 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐ MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐ T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate including the exemplary embodiments discussed above. [109] 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐
MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure may be used to prevent and/or treat inflammation and/or pain, such as for example inflammation and/or pain associated with inflammatory skeletal or muscular diseases or conditions. The disclosure provides a method for preventing and/or treating an inflammation and/or pain by administering to a subject in need thereof a therapeutically effective amount of 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐ PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐ Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐ Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure, including the exemplary embodiments discussed herein. Generally speaking, treatable “pain” includes nociceptive, neuropathic, and mix‐type. A method of the disclosure may reduce or alleviate the symptoms associated with inflammation, including but not limited to treating localized manifestation of inflammation characterized by acute or chronic swelling, pain, redness, increased temperature, or loss of function in some cases. A method of the disclosure may reduce or alleviate the symptoms of pain regardless of the cause of the pain, including but not limited to reducing pain of varying severity, i.e., mild, moderate and severe pain, acute pain and chronic pain. A method of the disclosure is effective in treating joint pain, muscle
pain, tendon pain, burn pain, and pain caused by inflammation such as rheumatoid arthritis. Skeletal or muscular diseases or conditions which may be treated include but are not limited to musculoskeletal sprains, musculoskeletal strains, tendinopathy, peripheral radiculopathy, osteoarthritis, joint degenerative disease, polymyalgia rheumatica, juvenile arthritis, gout, ankylosing spondylitis, psoriatic arthritis, systemic lupus erythematosus, costochondritis, tendonitis, bursitis, such as the common lateral epicondylitis (tennis elbow), medial epicondylitis (pitchers elbow) and trochanteric bursitis, temporomandibular joint syndrome, and fibromyalgia. [110] 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure may be used to modulate activity of a mitogen‐activated protein kinase (MAPK), comprising administering a composition of the invention. MAPKs provide a wide‐ ranging signaling cascade that allow cells to quickly respond to biotic and abiotic stimuli. Exemplary MAPKs include, but are not limited to, Tropomyosin Receptor Kinase A (TrkA), P38‐alpha, and c‐Jun N‐Terminal Kinase 3 (JNK3). TrkA is a high affinity catalytic receptor of nerve growth factor (NGF) protein. TrkA regulates NGF response, influencing neuronal differentiation and outgrowth as well as programmed cell death. p38‐alpha is involved with the regulation of pro‐inflammatory cytokines, including TNF‐a. In the central nervous system, p38‐alpha regulates neuronal death and neurite degeneration, and it is a common target of Alzheimer's disease therapies. JNK3 is a neuronal‐specific protein isoform of the JNKs. It is involved with the regulation of apoptosis. JNK3 also plays a role in modulating the response of cytokines, growth factors, and oxidative stress. [111] As used herein, the term “modulating activity of a mitogen‐activated protein kinase” refers to changing, manipulating, and/or adjusting the activity of a mitogen‐activated protein kinase. In one
embodiment, modulating the activity of a MAPK can influence neural health, neurogenesis, neural growth and differentiation, and neurodegenerative diseases. [112] 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure may be used to modulate neurogenesis, comprising administering a composition of the invention. As used herein, the term “modulating neurogenesis” refers to changing, manipulating, and/or adjusting the growth and development of neural tissue. In one embodiment, neurogenesis comprises adult neurogenesis, in which new neural stem cells are generated from neural stem cells in an adult animal. In one embodiment, modulating neurogenesis comprises increasing and/or enhancing the rate at which new neural tissue is developed. [113] 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate,
crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure may be used to modulate neurite outgrowth, comprising administering a composition of the invention. As used herein, the term “modulating neurite outgrowth” refers to changing, manipulating, and/or adjusting the growth and development of neural projections, or “neurites.” In one embodiment, neurogenesis comprises modulating the growth of new neurites, the number of neurites per neuron, and/or neurite length. In one embodiment, modulating neurite outgrowth comprises increasing and/or enhancing the rate and/or length at which neurites develop. [114] 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure may be used to prevent and/or treat sexual health disorders including, but not limited to, hypoactive sexual desire disorder, hyperactive sexual desire disorder, orgasmic disorder, arousal disorder, vaginismus, and dyspareunia. In some embodiments, the disorder is a male sexual dysfunction disorder. In some embodiments, the disorder is a female sexual dysfunction disorder. [115] 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐
MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure may be used to prevent and/or treat women’s health disorders including, but not limited to, menstrual cramping, dysmenorrhea, post‐hysterectomy pain, vaginal or vulvar vestibule mucosa disorder, menopausal‐related disorders, vaginal atrophy, or vulvar vestibulitis. [116] Compositions [117] The disclosure also relates to compositions comprising an effective amount of 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐ MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐ T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate and an excipient (e.g., a pharmaceutically‐acceptable excipient). In another embodiment, the disclosure also relates to pharmaceutical compositions comprising a therapeutically effective amount of 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐ MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline
5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐ T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate and a pharmaceutically acceptable excipient (also known as a pharmaceutically acceptable carrier). As discussed above, 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐ PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐ Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐ Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure may be, for example, therapeutically useful to prevent and/or treat the psychological disorders, brain disorders, pain, and inflammation as well as the other disorders described herein. [118] A composition or a pharmaceutical composition of the disclosure may be in any form which contains 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T
hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate. The composition may be, for example, a tablet, capsule, liquid suspension, injectable, topical, or transdermal. The compositions generally contain, for example, about 1% to about 99% by weight of 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐ PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐ Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐ Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure and, for example, 99% to 1% by weight of at least one suitable pharmaceutically acceptable excipient. In one embodiment, the composition may be between about 5% and about 75% by weight of 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐ PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐ Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐ Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT
fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure, with the rest being at least one suitable pharmaceutically acceptable excipient or at least one other adjuvant, as discussed below. [119] Published US applications US 2018/0221396 A1 and US 2019/0142851 A1 disclose compositions comprising a combination of a first purified psilocybin derivative with a second purified psilocybin derivative, with one or two purified cannabinoids or with a purified terpene. Various ratios of these components in the composition are also disclosed. The disclosures of US 2018/0221396 A1 and US 2019/0142851 A1 are incorporated herein by reference. According to this disclosure, 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐ PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐ Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐ Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure may be used as the “first purified psilocybin derivative” in the compositions described in US 2018/0221396 A1 and US 2019/0142851 A1. Accordingly, this disclosure provides a composition comprising: a first component comprising 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐ MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT
hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐ T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure; at least one second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, and (d) a purified terpene; and at least one pharmaceutically‐acceptable excipient or at least one other adjuvant. Such a composition may be a pharmaceutical composition wherein the components are present individually in therapeutically effective amounts or by combination in a therapeutically effective amount to treat a disease, disorder, or condition as described herein. [120] When used in such compositions as a first component comprising 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐ MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐ T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure with a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, and (d) a purified terpene, the compositions
represent particular embodiments of the invention. Compositions having as a first component 5‐ MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure with a second component selected from at least one of (e) an adrenergic drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone represent additional particular embodiments of the invention represented by the compositions having 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐ MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐ 5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐ T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate according to the disclosure. In some embodiments, the first and second components can be administered at the same time (e.g.,
together in the same composition), or at separate times over the course of treating a patient in need thereof. Such a composition may be a pharmaceutical composition wherein the components are present individually in therapeutically effective amounts or by combination in a therapeutically effective amount to treat a disease, disorder, or condition as described herein. [121] Within the context of this disclosure, the term “purified” means separated from other materials, such as plant or fungal material, e.g., protein, chitin, cellulose, or water. In one embodiment, the term “purified” refers to a compound substantially free of other materials. In one embodiment, the term “purified” refers to a compound that is substantially free from a second tryptamine compound. In one embodiment, the term “purified” refers to a compound substantially free from histidine. In one embodiment, the term “purified” refers to a compound substantially free from a biological material, such as mold, fungus, plant matter, or bacteria. In one embodiment, the term “purified” refers to a compound substantially free from a paralytic. [122] In one embodiment, the term “purified” refers to a compound which has been separated from other compounds that are typically co‐extracted when the purified compound is extracted from a naturally occurring organism. In one embodiment, a “purified” psilocybin derivative is partially or completely isolated from other psilocybin derivatives present in a source material, such as a psilocybin‐containing mushroom. In one example, “purified” baeocystin is substantially free from psilocybin and/or psilocin. By contrast, traditional psilocybin mushroom extracts (aka crude extracts or fruit body extracts) would be expected to contain an unpredictable and varying amount of psilocybin, psilocin, baeocystin, norbaeocystin, salts thereof, or combinations thereof. Other examples of unpurified psilocybin derivatives would include mycelium containing psilocybin derivatives and/or naturally occurring fungal material such as biological material and/or structural material such as chitin. Similarly, the term “cannabis extracts'' or “cannabinoid extracts” traditionally refers to whole plants (aka crude or full spectrum extracts) which have not been subjected to further purification to eliminate unwanted molecules that naturally occur in the cannabis plant. For example, a “cannabis extract comprising cannabidiol” could be expected to include cannabidiol (aka “CBD”) and also varying amounts of other compounds, including cannabinoids, terpenes, and other biological material. [123] In one embodiment, the term “purified” refers to a compound or composition that has been crystallized. [124] In one embodiment, the term “purified” refers to a compound or composition that has been chromatographed, for example by gas chromatography, liquid chromatography (e.g., LC, HPLC, etc.), etc.
[125] In one embodiment, the term “purified” refers to a compound or composition that has been distilled. [126] In one embodiment, the term “purified” refers to a compound or composition that has been sublimed. [127] In one embodiment, the term “purified” refers to a compound or composition that has been subject to two or more steps chosen from crystallization, chromatography, distillation, or sublimation. [128] In one embodiment, the term “purified” refers to a compound that is between 80‐100% pure. [129] In one embodiment, the term “purified” refers to a compound that is between 90‐100% pure. [130] In one embodiment, the term “purified” refers to a compound that is between 95‐100% pure. [131] In one embodiment, the term “purified” refers to a compound that is between 99‐100% pure. [132] In one embodiment, the term “purified” refers to a compound that is between 99.9‐100% pure. [133] A serotonergic drug refers to a compound that binds to, blocks, or otherwise influences (e.g., via an allosteric reaction) activity at a serotonin receptor as described in paragraphs [0245]‐[0253] of US 2018/0221396 A1 and [0305]‐[0311] US 2019/0142851 A1 as well as the disclosed exemplary embodiments. Exemplary psilocybin derivatives include but are not limited to psilocybin itself and the psilocybin derivatives described in paragraphs [0081]‐[0109] of US 2018/0221396 A1 and [0082]‐ [0110] US 2019/0142851 A1 as well as the disclosed exemplary embodiments. Exemplary cannabinoids include but are not limited to the cannabinoids described in paragraphs [0111]‐[0145] of US 2018/0221396 A1 and [0112]‐[0146] US 2019/0142851 A1 as well as the disclosed exemplary embodiments. Exemplary terpenes include but are not limited to the terpenes described in paragraphs [0160]‐[0238] of US 2018/0221396 A1 and [0161]‐[0300] US 2019/0142851 A1 as well as the disclosed exemplary embodiments. [134] A pharmaceutical formulation of the disclosure may comprise, consist essentially of, or consist of (a) 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐ PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐ Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐
Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure and (b) at least one second active compound selected from a serotonergic drug, a purified psilocybin derivative, a purified cannabinoid, a purified terpene, an adrenergic drug, a dopaminergic drug, a monoamine oxidase inhibitor, a purified erinacine, and a purified hericenone, and (c) a pharmaceutically acceptable excipient. In some embodiments, 5‐MeO‐ T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate and the second active compound(s) are each present in a therapeutically effective amount using purposefully engineered and unnaturally occurring molar ratios. Exemplary molar ratios of 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T
hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure to the second active compound in a composition of the disclosure include but are not limited to from about 0.1:100 to about 100:0.1, from about 1:100 to about 100:1, from about 1:50 to about 50:1, from about 1:25 to about 25:1, from about 1:20 to about 20:1, from about 1:10 to about 10:1, from about 1:5 to about 5:1, from about 1:2 to about 2:1 or may be about 1:1. [135] A pharmaceutical formulation of the disclosure may comprise a composition containing 5‐ MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure and a serotonergic drug, a purified psilocybin derivative, a purified cannabinoid, or a purified terpene, each present in a therapeutically effective amount using purposefully engineered and unnaturally occurring molar ratios. Published US applications US 2018/0221396 A1 and US 2019/0142851 A1 disclose compositions comprising a combination of a purified psilocybin derivative with a second purified psilocybin derivative, with one or two purified
cannabinoids or with a purified terpene. According to this disclosure composition containing 5‐MeO‐ T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure may be used in place of a “purified psilocybin derivative” in the compositions described in US 2018/0221396 A1 and US 2019/0142851 A1. Accordingly, the disclosure provides a pharmaceutical formulation comprising as (a) 5‐MeO‐T fumarate, crystalline 5‐ MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐ T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure and at least one second component selected from (a) a purified psilocybin derivative, (b) a purified cannabinoid, and (c) a purified terpene; and at least one pharmaceutically‐acceptable excipient or at
least one other adjuvant, as described herein. Such a composition may be a pharmaceutical composition wherein the components are present individually in therapeutically effective amounts or by combination in a therapeutically effective amount to treat a disease, disorder, or condition as described herein. [136] A serotonergic drug refers to a compound that binds to, blocks, or otherwise influences (e.g., via an allosteric reaction) activity at a serotonin receptor as described in paragraphs [0245]‐[0253] of US 2018/0221396 A1 and [0305]‐[0311] US 2019/0142851 A1 as well as the disclosed exemplary embodiments. Some exemplary serotonergic drugs include SSRIs and SNRIs. Some examples of specific serotonergic drugs include the following molecules, including any salts, solvates, or polymorphs thereof: 6‐allyl‐N,N‐diethyl‐NL; N,N‐dibutyl‐T; N,N‐diethyl‐T; N,N‐diisopropyl‐T; 5‐ methyoxy‐alpha‐methyl‐T; N,N‐dimethyl‐T; 2,alpha‐dimethyl‐T; alpha,N‐dimethyl‐T; N,N‐dipropyl‐T; N‐ethyl‐N‐isopropyl‐T; alpha‐ethyl‐T; 6‐N,N‐Triethyl‐NL; 3,4‐dihydro‐7‐methoxy‐1‐methyl‐C; 7‐ methyoxy‐1‐methyl‐C; N,N‐dibutyl‐4‐hydroxy‐T; N,N‐diethyl‐4‐hydroxy‐T; N,N‐diisopropyl‐4‐hydroxy‐ T; N,N‐dimethyl‐4‐hydroxy‐T; N,N‐dimethyl‐5‐hydroxy‐T; N, N‐dipropyl‐4‐hydroxy‐T; N‐ethyl‐4‐ hydroxy‐N‐methyl‐T; 4‐hydroxy‐N‐isopropyl‐N‐methyl‐T; 4‐hydroxy‐N‐methyl‐N‐propyl‐T; 4‐hydroxy‐ N,N‐tetramethylene‐T; ibogaine; N,N‐diethyl‐L; N‐butyl‐N‐methyl‐T; N,N‐diisopropyl‐4,5‐ methylenedioxy‐T; N,N‐diisopropyl‐5,6‐methylenedioxy‐T; N,N‐dimethyl‐4,5‐methylenedioxy‐T; N,N‐ dimethyl‐5,6‐methylenedioxy‐T; N‐isopropyl‐N‐methyl‐5,6‐methylenedioxy‐T; N,N‐diethyl‐2‐methyl‐ T; 2‐N,N‐trimethyl‐T; N‐acetyl‐5‐methoxy‐T; N,N‐diethyl‐5‐methoxy‐T; N,N‐diisopropyl‐5‐methoxy‐T; 5‐methoxy‐N,N‐dimethyl‐T; N‐isopropyl‐4‐methoxy‐N‐methyl‐T; N‐isopropyl‐5‐methoxy‐N‐methyl‐T; 5,6‐dimethoxy‐N‐isopropyl‐N‐methyl‐T; 5‐methoxy‐N‐methyl‐T; 5‐methoxy‐N,N‐tetramethylene‐T; 6‐methoxy‐1‐methyl‐1,2,3,4‐tetrahydro‐C; 5‐methoxy‐2‐N,N‐trimethyl‐T; N,N‐dimethyl‐5‐ methylthio‐T; N‐isopropyl‐N‐methyl‐T; alpha‐methyl‐T; N‐ethyl‐T; N‐methyl‐T; 6‐propyl‐N L; N,N‐ tetramethylene‐T; tryptamine; 7‐methoxy‐1‐methyl‐1,2,3,4‐tetrahydro‐C; and alpha,N‐dimethyl‐5‐ methoxy‐T. For additional information regarding these compounds see Shulgin, A. T., & Shulgin, A. (2016). Tihkal: The Continuation. Berkeley, Calif.: Transform Press. In one embodiment, a serotonergic drug is chosen from alprazolam, amphetamine, aripiprazole, azapirone, a barbiturate, bromazepam, bupropion, buspirone, a cannabinoid, chlordiazepoxide, citalopram, clonazepam, clorazepate, dextromethorphan, diazepam, duloxetine, escitalopram, fluoxetine, flurazepam, fluvoxamine, lorazepam, lysergic acid diethylamide, lysergamide, 3,4‐ methylenedioxymethamphetamine, milnacipran, mirtazapine, naratriptan, paroxetine, pethidine, phenethylamine, psicaine, oxazepam, reboxetine, serenic, serotonin, sertraline, temazepam, tramadol, triazolam, a tryptamine, venlafaxine, vortioxetine, and/or derivatives thereof. In an exemplary embodiment, the serotonergic drug is 3,4‐methylenedioxymethamphetamine.
[137] Exemplary psilocybin derivatives include but are not limited to psilocybin itself and the psilocybin derivatives described in paragraphs [0081]‐[0109] of US 2018/0221396 A1 and [0082]‐ [0110] US 2019/0142851 A1 as well as the disclosed exemplary embodiments, incorporated here by reference. In one embodiment, the compositions disclosed herein comprise one or more purified psilocybin derivatives chosen from: [3‐(2‐dimethylaminoethyl)‐1H‐indol‐4‐yl] dihydrogen phosphate; 4‐hydroxytryptamine; 4‐hydroxy‐N,N‐dimethyltryptamine; [3‐(2‐methylaminoethyl)‐1H‐indol‐4‐yl] dihydrogen phosphate; 4‐hydroxy‐N‐methyltryptamine; [3‐(aminoethyl)‐1H‐indol‐4‐yl] dihydrogen phosphate; [3‐(2‐trimethylaminoethyl)‐1H‐indol‐4‐yl] dihydrogen phosphate; and 4‐hydroxy‐N,N,N‐ trimethyltryptamine. [138] Exemplary cannabinoids include but are not limited to the cannabinoids described in paragraphs [0111]‐[0145] of US 2018/0221396 A1 and [0112]‐[0146] US 2019/0142851 A1 as well as the disclosed exemplary embodiments. Examples of cannabinoids within the context of this disclosure include the following molecules: cannabichromene (CBC); cannabichromenic acid (CBCA); cannabichromevarin (CBCV); cannabichromevarinic acid (CBCVA); cannabicyclol (CBL); cannabicyclolic acid (CBLA); cannabicyclovarin (CBLV); cannabidiol (CBD); cannabidiol monomethylether (CBDM); cannabidiolic acid (CBDA); cannabidiorcol (CBD‐C1); cannabidivarin (CBDV); cannabidivarinic acid (CBDVA); cannabielsoic acid B (CBEA‐B); cannabielsoin (CBE); cannabielsoin acid A (CBEA‐A); cannabigerol (CBG); cannabigerol monomethylether (CBGM); cannabigerolic acid (CBGA); cannabigerolic acid monomethylether (CBGAM); cannabigerovarin (CBGV); cannabigerovarinic acid (CBGVA); cannabinodiol (CBND); cannabinodivarin (CBVD); cannabinol (CBN); cannabinol methylether (CBNM); cannabinol‐C2 (CBN‐C2); cannabinol‐C4 (CBN‐ C4); cannabinolic acid (CBNA); cannabiorcol (CBN‐C1); cannabivarin (CBV); cannabitriol (CBT); cannabitriolvarin (CBTV); 10‐ethoxy‐9‐hydroxy‐delta‐6a‐tetrahydrocannabinol; cannabicitran (CBTC); cannabiripsol (CBR); 8,9‐dihydroxy‐delta‐6a‐tetrahydrocannabinol; delta‐8‐tetrahydrocannabinol (Δ8‐THC); delta‐8‐tetrahydrocannabinolic acid (Δ8‐THCA); delta‐9‐tetrahydrocannabinol (THC); delta‐ 9‐tetrahydrocannabinol‐C4 (THC‐C4); delta‐9‐tetrahydrocannabinolic acid A (THCA‐A); delta‐9‐ tetrahydrocannabinolic acid B (THCA‐B); delta‐9‐tetrahydrocannabinolic acid‐C4 (THCA‐C4); delta‐9‐ tetrahydrocannabiorcol (THC‐C1); delta‐9‐tetrahydrocannabiorcolic acid (THCA‐C1); delta‐9‐ tetrahydrocannabivarin (THCV); delta‐9‐tetrahydrocannabivarinic acid (THCVA); 10‐oxo‐delta‐6a‐ tetrahydrocannabinol (OTHC); cannabichromanon (CBCF); cannabifuran (CBF); cannabiglendol; delta‐9‐cis‐tetrahydrocannabinol (cis‐THC); trihydroxy‐delta‐9‐tetrahydrocannabinol (triOH‐THC); dehydrocannabifuran (DCBF); and 3,4,5,6‐tetrahydro‐7‐hydroxy‐alpha‐alpha‐2‐trimethyl‐9‐n‐propyl‐ 2,6‐methano‐2H‐1‐benzoxocin‐5‐methanol. In one embodiment, the purified cannabinoid is chosen
from THC, THCA, THCV, THCVA, CBC, CBCA, CBCV, CBCVA, CBD, CBDA, CBDV, CBVD, CBDVA, CBG, CBGA, CBGV, or CBGVA. [139] Exemplary terpenes include but are not limited to the terpenes described in paragraphs [0160]‐[0238] of US 2018/0221396 A1 and [0161]‐[0300] US 2019/0142851 A1 as well as the disclosed exemplary embodiments. In one embodiment, a purified terpene is chosen from acetanisole, acetyl cedrene, anethole, anisole, benzaldehyde, bornyl acetate, borneol, cadinene, cafestol, caffeic acid, camphene, camphor, capsaicin, carene, carotene, carvacrol, carvone, caryophyllene, caryophyllene, caryophyllene oxide, cedrene, cedrene epoxide, cecanal, cedrol, cembrene, cinnamaldehyde, cinnamic acid, citronellal, citronellol, cymene, eicosane, elemene, estragole, ethyl acetate, ethyl cinnamate, ethyl maltol, eucalyptol/1,8‐cineole, eudesmol, eugenol, euphol, farnesene, farnesol, fenchone, geraniol, geranyl acetate, guaia‐1(10),11‐diene, guaiacol, guaiol, guaiene, gurjunene, herniarin, hexanaldehyde, hexanoic acid, humulene, ionone, ipsdienol, isoamyl acetate, isoamyl alcohol, isoamyl formate, isoborneol, isomyrcenol, isoprene, isopulegol, isovaleric acid, lavandulol, limonene, gamma‐linolenic acid, linalool, longifolene, lycopene, menthol, methyl butyrate, 3‐mercapto‐2‐methylpentanal, beta‐mercaptoethanol, mercaptoacetic acid, methyl salicylate, methylbutenol, methyl‐2‐methylvalerate, methyl thiobutyrate, myrcene, gamma‐ muurolene, nepetalactone, nerol, nerolidol, neryl acetate, nonanaldehyde, nonanoic acid, ocimene, octanal, octanoic acid, pentyl butyrate, phellandrene, phenylacetaldehyde, phenylacetic acid, phenylethanethiol, phytol, pinene, propanethiol, pristimerin, pulegone, retinol, rutin, sabinene, squalene, taxadiene, terpineol, terpine‐4‐ol, terpinolene, thujone, thymol, umbelliferone, undecanal, verdoxan, or vanillin. In one embodiment, a purified terpene is chosen from bornyl acetate, alpha‐ bisabolol, borneol, camphene, camphor, carene, caryophyllene, cedrene, cymene, elemene, eucalyptol, eudesmol, farnesene, fenchol, geraniol, guaiacol, humulene, isoborneol, limonene, linalool, menthol, myrcene, nerolidol, ocimene, phellandrene, phytol, pinene, pulegone, sabinene, terpineol, terpinolene, or valencene. [140] As used herein, the term “adrenergic drug” refers to a compound that binds, blocks, or otherwise influences (e.g., via an allosteric reaction) activity at an adrenergic receptor. In one embodiment, an adrenergic drug binds to an adrenergic receptor. In one embodiment, an adrenergic drug indirectly affects an adrenergic receptor, e.g., via interactions affecting the reactivity of other molecules at the adrenergic receptor. In one embodiment, an adrenergic drug is an agonist, e.g., a compound activating an adrenergic receptor. In one embodiment, an adrenergic drug is an antagonist, e.g., a compound binding but not activating an adrenergic receptor, e.g., blocking a receptor. In one embodiment, an adrenergic drug is an effector molecule, e.g., a compound binding to an enzyme for allosteric regulation. In one embodiment, an adrenergic drug acts (either directly
or indirectly) at more than one type of receptor (e.g., 5HT, dopamine, adrenergic, acetylcholine, etc.). [141] In one embodiment, an adrenergic drug is an antidepressant. In one embodiment, an adrenergic drug is a norepinephrine transporter inhibitor. In one embodiment, an adrenergic drug is a vesicular monoamine transporter inhibitor. In one embodiment, an adrenergic drug is chosen from adrenaline, agmatine, amoxapine, aptazapine, atomoxetine, bupropion, clonidine, doxepin, duloxetine, esmirtazpine, mianserin, ketanserin, mirabegron, mirtazapine, norepinephrine, phentolamine, phenylephrine, piperoxan, reserpine, ritodrine, setiptiline, tesofensine, timolol, trazodone, trimipramine, or xylazine. [142] As used herein, the term “dopaminergic drug” refers to a compound that binds, blocks, or otherwise influences (e.g., via an allosteric reaction) activity at a dopamine receptor. In one embodiment, a dopaminergic drug binds to a dopamine receptor. In one embodiment, a dopaminergic drug indirectly affects a dopamine receptor, e.g., via interactions affecting the reactivity of other molecules at the dopamine receptor. In one embodiment, a dopaminergic drug is an agonist, e.g., a compound activating a dopamine receptor. In one embodiment, a dopaminergic drug is an antagonist, e.g., a compound binding but not activating a dopamine receptor, e.g., blocking a receptor. In one embodiment, a dopaminergic drug is an effector molecule, e.g., a compound binding to an enzyme for allosteric regulation. In one embodiment, a dopaminergic drug acts (either directly or indirectly) at more than one type of receptor (e.g., 5HT, dopamine, adrenergic, acetylcholine, etc.). [143] In one embodiment, a dopaminergic drug is a dopamine transporter inhibitor. In one embodiment, a dopaminergic drug is a vesicular monoamine transporter inhibitor. In one embodiment, a dopaminergic drug is chosen from amineptine, apomorphine, benzylpiperazine, bromocriptine, cabergoline, chlorpromazine, clozapine, dihydrexidine, domperidone, dopamine, fluphenazine, haloperidol, ketamine, loxapine, methamphetamine, olanzapine, pemoline, perphenazine, pergolide, phencyclidine, phenethylamine, phenmetrazine, pimozide, piribedil, a psychostimulant, reserpine, risperidone, ropinirole, tetrabenazine, or thioridazine. [144] As used herein, the term “monoamine oxidase inhibitor” (MAOI) refers to a compound that blocks the actions of monoamine oxidase enzymes. In one embodiment, a MAOI inhibits the activity of one or both monoamine oxidase A and monoamine oxidase B. In one embodiment a MAOI is a reversible inhibitor of monoamine oxidase A. In one embodiment a MAOI is a drug chosen from isocarboxazid, phenelzine, or tranylcypromine. In one embodiment, a MAOI is β‐carboline, pinoline, harmane, harmine, harmaline, harmalol, tetrahydroharmine, 9‐methyl‐β‐carboline, or 3‐carboxy‐ tetrahydrononharman.
[145] In one embodiment, the compositions and methods disclosed herein include one or more purified erinacine molecules. In one embodiment, the compositions and methods disclosed herein comprise purified erinacine A. In one embodiment, the compositions and methods disclosed herein comprise erinacine B. In one embodiment, the compositions and methods disclosed herein comprise erinacine C. In one embodiment, the compositions and methods disclosed herein comprise erinacine D. In one embodiment, the compositions and methods disclosed herein comprise erinacine E. In one embodiment, the compositions and methods disclosed herein comprise erinacine F. In one embodiment, the compositions and methods disclosed herein comprise erinacine G. In one embodiment, the compositions and methods disclosed herein comprise erinacine H. In one embodiment, the compositions and methods disclosed herein comprise erinacine I. In one embodiment, the compositions and methods disclosed herein comprise erinacine J. In one embodiment, the compositions and methods disclosed herein comprise erinacine K In one embodiment, the compositions and methods disclosed herein comprise erinacine P. In one embodiment, the compositions and methods disclosed herein comprise erinacine Q. In one embodiment, the compositions and methods disclosed herein comprise erinacine R. In one embodiment, the compositions and methods disclosed herein comprise erinacine S. [146] In one embodiment, the compositions and methods disclosed herein include one or more purified hericenone molecules. In one embodiment, the compositions and methods disclosed herein comprise purified hericenone A. In one embodiment, the compositions and methods disclosed herein comprise purified hericenone B. In one embodiment, the compositions and methods disclosed herein comprise purified hericenone C. In one embodiment, the compositions and methods disclosed herein comprise purified hericenone D. In one embodiment, the compositions and methods disclosed herein comprise purified hericenone E. In one embodiment, the compositions and methods disclosed herein comprise purified hericenone F. In one embodiment, the compositions and methods disclosed herein comprise purified hericenone G. In one embodiment, the compositions and methods disclosed herein comprise purified hericenone H. [147] Exemplary compositions of 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐ MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐ 5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐ T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT
hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure and a second compound selected from a serotonergic drug, a purified psilocybin derivative, a purified cannabinoid, a purified terpene, an adrenergic drug, a dopaminergic drug, a monoamine oxidase inhibitor, a purified erinacine, and a purified hericenone in exemplary molar ratios are shown in Tables 1a and 1b. 5‐ MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure may be any one of the exemplary embodiments described above including the crystalline forms as disclosed herein. Table 1a






Table 1b

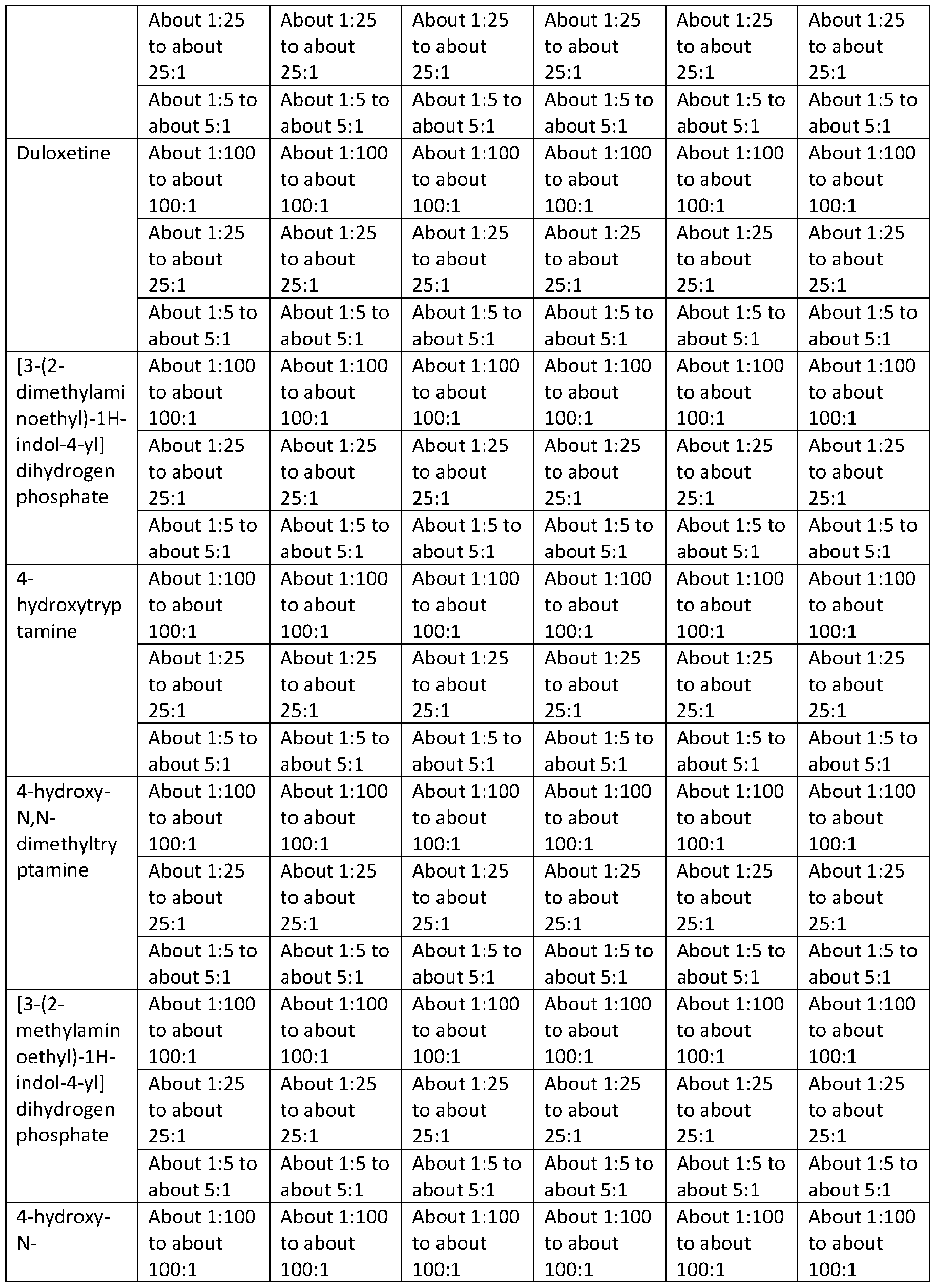

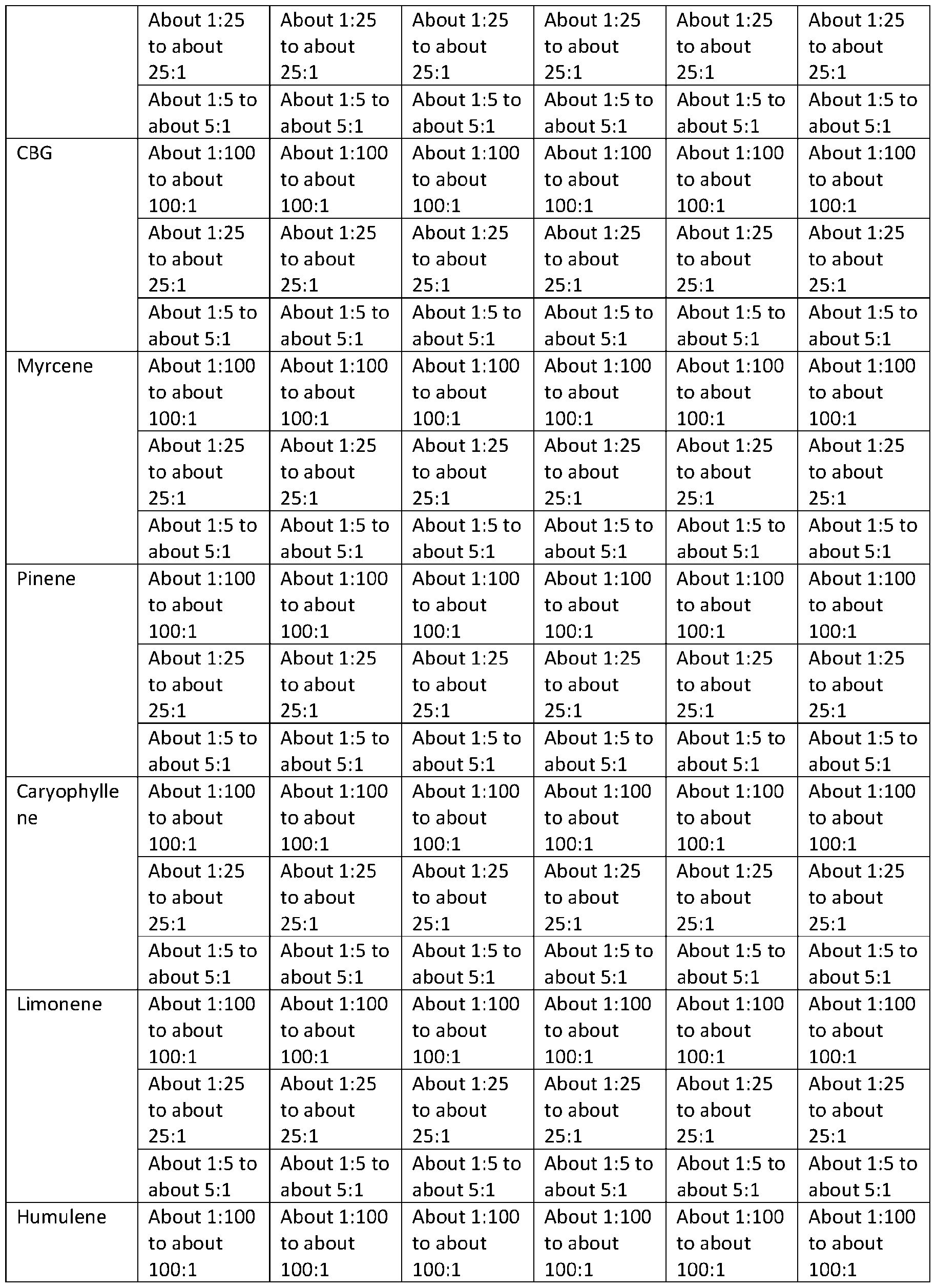


[148] Exemplary pharmaceutical compositions of 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐ MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐ 5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐ T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure and a second compound selected from a serotonergic drug, a purified psilocybin derivative, a purified cannabinoid, a purified terpene, an adrenergic drug, a dopaminergic drug, a monoamine oxidase inhibitor, a purified erinacine, and a purified hericenone and an excipient with exemplary molar ratios of 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐ MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐
T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate to the second compound are shown in Tables 2a and 2b. 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐ MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐ 5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐ T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure may be any one of the exemplary embodiments described above including the crystalline forms as disclosed herein. Table 2a

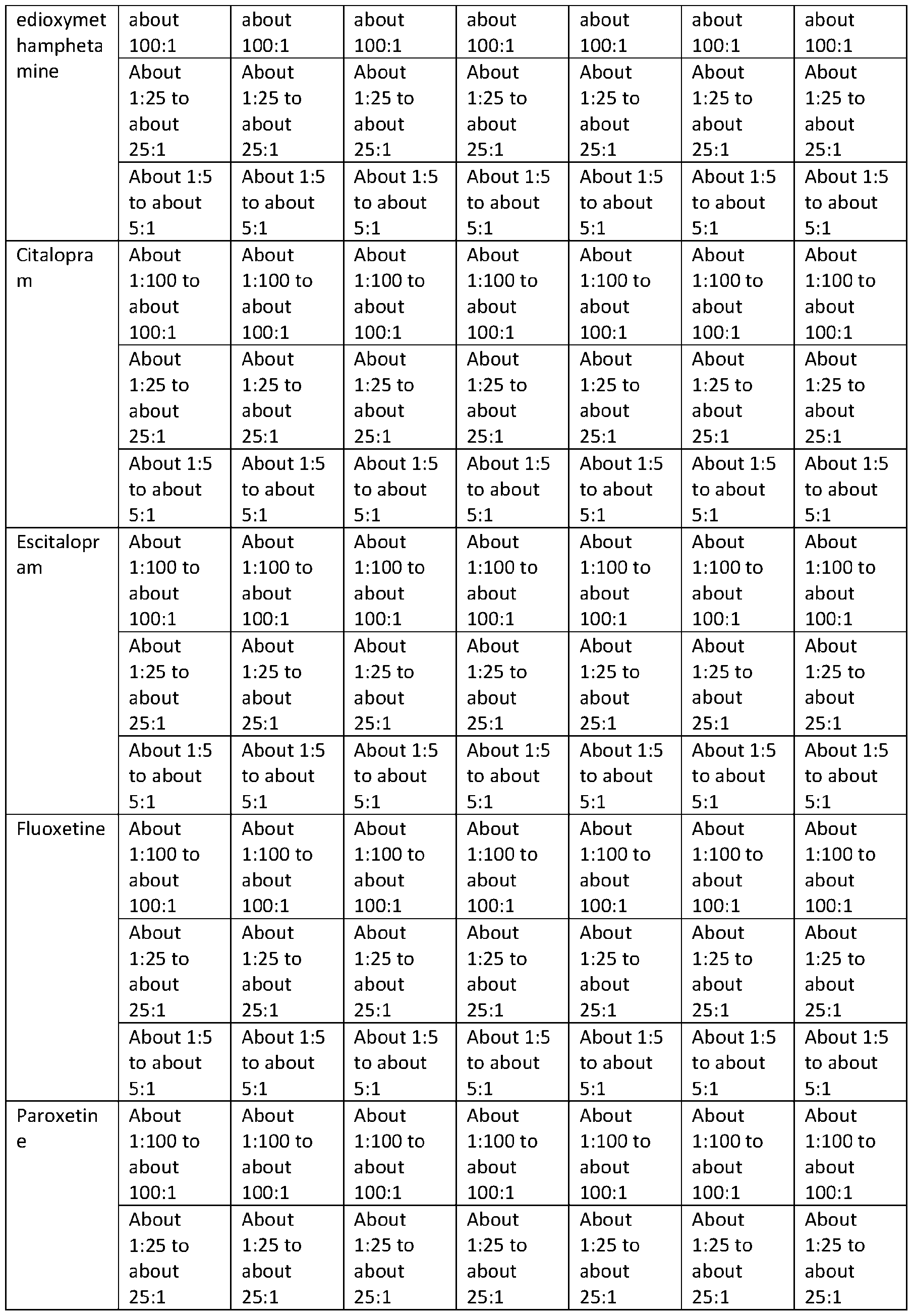






Table 2b


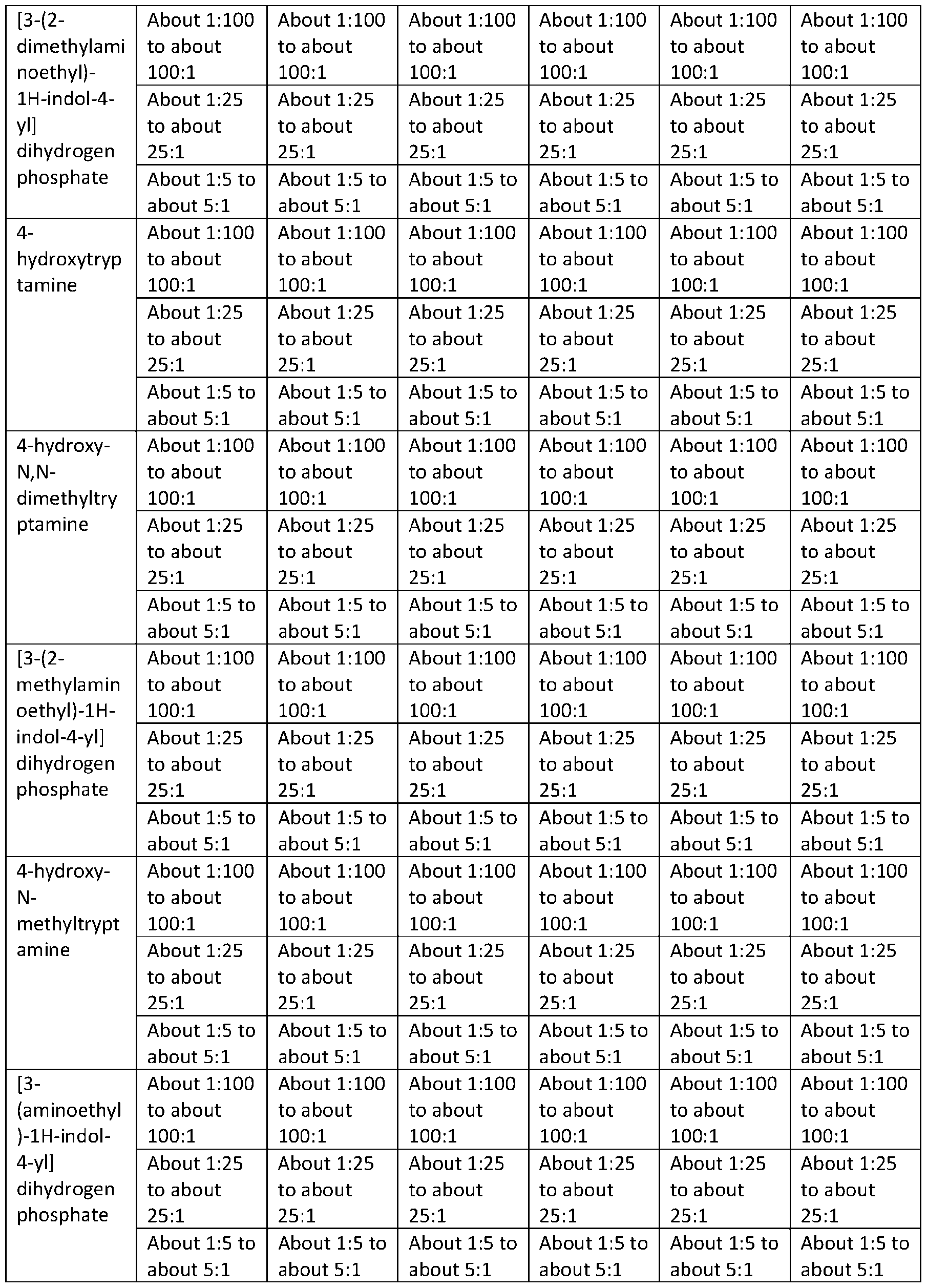



[149] An “effective amount” or a “therapeutically effective amount” of 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐ MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate,
crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐ T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure is generally in the range of about 0.1 to about 100 mg daily (oral dose), of about 0.1 to about 50 mg daily (oral dose), of about 0.25 to about 25 mg daily (oral dose), of about 0.1 to about 5 mg daily (oral dose), or of about 0.5 to about 2.5 mg daily (oral dose). The actual amount required for treatment of any particular patient may depend upon a variety of factors including, for example, the disease being treated and its severity; the specific pharmaceutical composition employed; the age, body weight, general health, sex, and diet of the patient; the mode of administration; the time of administration; the route of administration; and the rate of excretion; the duration of the treatment; any drugs used in combination or coincidental with the specific compound employed; and other such factors well known in the medical arts. These factors are discussed in Goodman and Gilman’s “The Pharmacological Basis of Therapeutics,” Tenth Edition, A. Gilman, J. Hardman and L. Limbird, eds., McGraw‐Hill Press, 155‐173 (2001), which is incorporated herein by reference. 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐ MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐ T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure
and pharmaceutical compositions containing it may be used in combination with other agents that are generally administered to a patient being treated for psychological and other disorders discussed above. They may also be co‐formulated with one or more of such agents in a single pharmaceutical composition. [150] Depending on the type of pharmaceutical composition, the pharmaceutically acceptable carrier may be chosen from any one or a combination of carriers known in the art. The choice of the pharmaceutically acceptable carrier depends upon the pharmaceutical form and the desired method of administration to be used. Exemplary carriers include those that do not substantially alter the structure or activity of 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐ PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐ Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐ Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure, or produce undesirable biological effects or otherwise interact in a deleterious manner with any other component(s) of the pharmaceutical composition. [151] The pharmaceutical compositions of the disclosure may be prepared by methods know in the pharmaceutical formulation art, for example, see Remington’s Pharmaceutical Sciences, 18th Ed., (Mack Publishing Company, Easton, Pa., 1990), which is incorporated herein by reference. In a solid dosage form, 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐ PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐ Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐ Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT
fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure may be admixed with at least one pharmaceutically acceptable excipient such as, for example, sodium citrate or dicalcium phosphate or (a) fillers or extenders, such as, for example, starches, lactose, sucrose, glucose, mannitol, and silicic acid, (b) binders, such as, for example, cellulose derivatives, starch, alginates, gelatin, polyvinylpyrrolidone, sucrose, and gum acacia, (c) humectants, such as, for example, glycerol, (d) disintegrating agents, such as, for example, agar‐agar, calcium carbonate, potato or tapioca starch, alginic acid, croscarmellose sodium, complex silicates, and sodium carbonate, (e) solution retarders, such as, for example, paraffin, (f) absorption accelerators, such as, for example, quaternary ammonium compounds, (g) wetting agents, such as, for example, cetyl alcohol, and glycerol monostearate, magnesium stearate and the like, (h) adsorbents, such as, for example, kaolin and bentonite, and (i) lubricants, such as, for example, talc, calcium stearate, magnesium stearate, solid polyethylene glycols, sodium lauryl sulfate, or mixtures thereof. In the case of capsules, tablets, and pills, the dosage forms may also comprise buffering agents. In some embodiments, the excipient is not water. In some embodiments, the excipient is not a solvent (e.g., EtOH, diethyl ether, ethyl acetate, or hydrocarbon‐based solvents (e.g., hexanes). In some embodiments, the dosage form is substantially free of water and/or solvents, for example less than about 5% water by mass, less than 2% water by mass, less than 1% water by mass, less than 0.5% water by mass, or less than 0.1% water by mass. [152] Excipients or pharmaceutically acceptable adjuvants known in the pharmaceutical formulation art may also be used in the pharmaceutical compositions of the disclosure. These include, but are not limited to, preserving, wetting, suspending, sweetening, flavoring, perfuming, emulsifying, and dispensing agents. Prevention of the action of microorganisms may be ensured by inclusion of various antibacterial and antifungal agents, for example, parabens, chlorobutanol, phenol, sorbic acid, and the like. It may also be desirable to include isotonic agents, for example, sugars, sodium chloride, and the like. If desired, a pharmaceutical composition of the disclosure may also contain minor amounts of auxiliary substances such as wetting or emulsifying agents, pH buffering agents, antioxidants, and the like, such as, for example, citric acid, sorbitan monolaurate, triethanolamine oleate, butylated hydroxytoluene, etc.
[153] Solid dosage forms as described above may be prepared with coatings and shells, such as enteric coatings and others well known in the art. They may contain pacifying agents and can also be of such composition that they release the active compound or compounds in a certain part of the intestinal tract in a delayed manner. Non‐limiting examples of embedded compositions that may be used are polymeric substances and waxes. The active compounds may also be in microencapsulated form, if appropriate, with one or more of the above‐mentioned excipients. [154] Suspensions, in addition to the active compounds, may contain suspending agents, such as, for example, ethoxylated isostearyl alcohols, polyoxyethylene sorbitol and sorbitan esters, microcrystalline cellulose, aluminum metahydroxide, bentonite, agar‐agar and tragacanth, or mixtures of these substances, and the like. [155] Solid dosage forms for oral administration, which includes capsules, tablets, pills, powders, and granules, may be used. In such solid dosage forms, the active compound may be mixed with at least one inert, pharmaceutically acceptable excipient (also known as a pharmaceutically acceptable carrier). [156] Administration of 5‐MeO‐T fumarate, crystalline 5‐MeO‐T fumarate, tryptammonium hydromalate, crystalline tryptammonium hydromalate, tryptammonium hydrophthalate, crystalline tryptammonium hydrophthalate, 4‐MeO‐MiPT fumarate, crystalline 4‐MeO‐MiPT fumarate, 4‐HO‐ PiPT hydrofumarate, crystalline 4‐HO‐PiPT hydrofumarate, 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, crystalline 5‐Cl‐T∙N‐ Suc‐5‐Cl‐T, 5‐Cl‐T hydromaleate, crystalline 5‐Cl‐T hydromaleate, 5‐Cl‐T hydrophthalate, crystalline 5‐ Cl‐T hydrophthalate, 5‐Cl‐T malate, crystalline 5‐Cl‐T malate, 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline 5‐ MeO‐T∙N‐Suc‐5‐MeO‐T, 5‐Me‐T, crystalline 5‐Me‐T, 4‐HO‐MCPT fumarate, crystalline 4‐HO‐MCPT fumarate, 4‐HO‐DiPT hydrofumarate, crystalline 4‐HO‐DiPT hydrofumarate or specific crystalline forms thereof, such as crystalline form 1 of 5‐MeO‐T fumarate, crystalline form 1 of tryptammonium hydromalate, crystalline form 1 of tryptammonium hydrophthalate, crystalline form 1 of 4‐MeO‐ MiPT fumarate, crystalline form 1 of 4‐HO‐PiPT hydrofumarate, crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐ Cl‐T, crystalline form 1 of 5‐Cl‐T hydromaleate, crystalline form 1 of 5‐Cl‐T hydrophthalate, crystalline form 1 of 5‐Cl‐T malate, crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, crystalline form 1 of 5‐Me‐T, crystalline form 1 of 4‐HO‐MCPT fumarate, and crystalline form 1 of 4‐HO‐DiPT hydrofumarate of the disclosure in pure form or in an appropriate pharmaceutical composition may be carried out via any of the accepted modes of administration or agents for serving similar utilities. Thus, administration may be, for example, orally, buccally, nasally, parenterally (intravenous, intramuscular, or subcutaneous), topically, transdermally, intravaginally, intravesically, or intrasystemically, in the form of solid, semi‐solid, lyophilized powder, or liquid dosage forms, such as, for example, tablets, suppositories, pills, soft elastic and hard gelatin capsules, powders,
solutions, suspensions, or aerosols, or the like, such as, for example, in unit dosage forms suitable for simple administration of precise dosages. One route of administration may be oral administration, using a convenient daily dosage regimen that can be adjusted according to the degree of severity of the disease‐state to be treated. [157] Exemplary Embodiments of the Invention [158] E1. Bis(2‐(5‐methoxy‐1H‐indol‐3‐yl)ethan‐1‐aminium) (2E)‐but‐2‐enedioate (5‐ methoxytryptammonium fumarate). [159] E2. Crystalline bis(2‐(5‐methoxy‐1H‐indol‐3‐yl)ethan‐1‐aminium) (2E)‐but‐2‐enedioate (5‐ methoxytryptammonium fumarate). [160] E3. Crystalline form 1 of bis(2‐(5‐methoxy‐1H‐indol‐3‐yl)ethan‐1‐aminium) (2E)‐but‐2‐ enedioate (5‐methoxytryptammonium fumarate). [161] E4. Crystalline form 1 of 5‐methoxytryptammonium fumarate according to E3, characterized by at least one of: a monoclinic crystal system at a temperature of about 297 K; a P2
1/c space group at a temperature of about 297 K; unit cell dimensions a = 5.8070(3) Å, b = 24.2768(13) Å, c = 8.9334(5) Å, α = 90°, β = 103.360(2)°, and ɣ = 90°; an X‐ray powder diffraction pattern substantially similar to FIG. 30; or an X‐ray powder diffraction pattern characterized by at least two peaks selected from 7.3, 12.5, and 24.8 °2θ ± 0.2 °2θ. [162] E5. A composition comprising 5‐methoxytryptammonium fumarate according to E1 and an excipient. [163] E6. A composition comprising crystalline 5‐methoxytryptammonium fumarate according to any one of E2‐E4 and an excipient. [164] E7. A composition comprising 5‐methoxytryptammonium fumarate according to E1 as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone. [165] E8. A composition comprising crystalline 5‐methoxytryptammonium fumarate according to any one of E2‐E4 as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone.
[166] E9. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a therapeutically effective amount of 5‐ methoxytryptammonium fumarate according to E1. [167] E10. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline 5‐ methoxytryptammonium fumarate according to any one of E2‐E4. [168] E11. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a composition according to E5 or E7. [169] E12. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a composition according to E6 or E8. [170] E13. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a therapeutically effective amount of 5‐ methoxytryptammonium fumarate according to E1. [171] E14. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline 5‐ methoxytryptammonium fumarate according to any one of E2‐E4. [172] E15. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a composition according to E5 or E7. [173] E16. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a composition according to E6 or E8. [174] E17. 2‐(1H‐indol‐3‐yl)ethan‐1‐aminium 3‐carboxy‐2‐hydroxypropanoate (tryptammonium hydromalate). [175] E18. Crystalline 2‐(1H‐indol‐3‐yl)ethan‐1‐aminium 3‐carboxy‐2‐hydroxypropanoate (tryptammonium hydromalate) [176] E19. Crystalline form 1 of 2‐(1H‐indol‐3‐yl)ethan‐1‐aminium 3‐carboxy‐2‐hydroxypropanoate (tryptammonium hydromalate). [177] E20. Crystalline form 1 of tryptammonium hydromalate according to E19, characterized by at least one of: an orthorhombic crystal system at a temperature of about 297 K; a P2
12
12
1 space group at a temperature of about 297 K; unit cell dimensions a = 7.2012(5) Å, b = 8.2509(5) Å, c = 24.7541(16) Å, α = 90°, β = 90°, and ɣ = 90°; an X‐ray powder diffraction pattern substantially similar to FIG. 31; or
an X‐ray powder diffraction pattern characterized by at least two peaks selected from 7.1, 11.3, and 20.9 °2θ ± 0.2 °2θ. [178] E21. A composition comprising tryptammonium hydromalate according to E17 and an excipient. [179] E22. A composition comprising crystalline tryptammonium hydromalate according to any one of E18‐E20 and an excipient. [180] E23. A composition comprising tryptammonium hydromalate according to E17 as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone. [181] E24. A composition comprising crystalline tryptammonium hydromalate according to any one of E18‐E20 as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone. [182] E25. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a therapeutically effective amount of tryptammonium hydromalate according to E17. [183] E26. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline tryptammonium hydromalate according to any one of E18‐E20. [184] E27. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a composition according to E21 or E23. [185] E28. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a composition according to E22 or E24. [186] E29. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a therapeutically effective amount of tryptammonium hydromalate according to E17. [187] E30. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline tryptammonium hydromalate according to any one of E18‐E20. [188] E31. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a composition according to E21 or E23.
[189] E32. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a composition according to E22 or E24. [190] E33. 2‐(1H‐indol‐3‐yl)ethan‐1‐aminium 2‐carboxybenzoate (tryptammonium hydrophthalate). [191] E34. Crystalline 2‐(1H‐indol‐3‐yl)ethan‐1‐aminium 2‐carboxybenzoate (tryptammonium hydrophthalate). [192] E35. Crystalline form 1 of 2‐(1H‐indol‐3‐yl)ethan‐1‐aminium 2‐carboxybenzoate (tryptammonium hydrophthalate). [193] E36. Crystalline form 1 of tryptammonium hydrophthalate according to E35, characterized by at least one of: a monoclinic crystal system at a temperature of about 297 K; a C2/c space group at a temperature of about 297 K; unit cell dimensions a = 18.7823(11) Å, b = 5.7731(3) Å, c = 29.3825(19) Å, α = 90°, β = 90.617(2)°, and ɣ = 90°; an X‐ray powder diffraction pattern substantially similar to FIG. 32; or an X‐ray powder diffraction pattern characterized by at least two peaks selected from 6.0, 11.2, and 15.4 °2θ ± 0.2 °2θ. [194] E37. A composition comprising tryptammonium hydrophthalate according to E33 and an excipient. [195] E38. A composition comprising crystalline tryptammonium hydrophthalate according to any one of E34‐E36 and an excipient. [196] E39. A composition comprising tryptammonium hydrophthalate according to E33 as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone. [197] E40. A composition comprising crystalline tryptammonium hydrophthalate according to any one of E34‐E36 as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone. [198] E41. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a therapeutically effective amount of tryptammonium hydrophthalate according to E33.
[199] E42. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline tryptammonium hydrophthalate according to any one of E34‐E36. [200] E43. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a composition according to E37 or E39. [201] E44. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a composition according to E38 or E40. [202] E45. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a therapeutically effective amount of tryptammonium hydrophthalate according to E33. [203] E46. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline tryptammonium hydrophthalate according to any one of E34‐E36. [204] E47. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a composition according to E37 or E39. [205] E48. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a composition according to E38 or E40. [206] E49. Bis([2‐(4‐methoxy‐1H‐indol‐3‐yl)ethyl](methyl)(propan‐2‐yl)azanium) (2E)‐but‐2‐ enedioate (4‐methoxy‐N‐methyl‐N‐isopropyltryptammonium fumarate). [207] E50. Crystalline bis([2‐(4‐methoxy‐1H‐indol‐3‐yl)ethyl](methyl)(propan‐2‐yl)azanium) (2E)‐ but‐2‐enedioate (4‐methoxy‐N‐methyl‐N‐isopropyltryptammonium fumarate). [208] E51. Crystalline form 1 of bis([2‐(4‐methoxy‐1H‐indol‐3‐yl)ethyl](methyl)(propan‐2‐ yl)azanium) (2E)‐but‐2‐enedioate (4‐methoxy‐N‐methyl‐N‐isopropyltryptammonium fumarate). [209] E52. Crystalline form 1 of 4‐methoxy‐N‐methyl‐N‐isopropyltryptammonium fumarate according to E51, characterized by at least one of: a monoclinic crystal system at a temperature of about 297 K; a P2
1/n space group at a temperature of about 297 K; unit cell dimensions a = 9.1438(3) Å, b = 13.8957(6) Å, c = 13.6658(6) Å, α = 90°, β = 94.6140(10)°, and ɣ = 90°; an X‐ray powder diffraction pattern substantially similar to FIG.33; or an X‐ray powder diffraction pattern characterized by at least two peaks selected from 9.1, 14.5, and 16.0 °2θ ± 0.2 °2θ. [210] E53. A composition comprising 4‐methoxy‐N‐methyl‐N‐isopropyltryptammonium fumarate according to E49 and an excipient.
[211] E54. A composition comprising crystalline 4‐methoxy‐N‐methyl‐N‐isopropyltryptammonium fumarate according to any one of E50‐E52 and an excipient. [212] E55. A composition comprising 4‐methoxy‐N‐methyl‐N‐isopropyltryptammonium fumarate according to E49 as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone. [213] E56. A composition comprising crystalline 4‐methoxy‐N‐methyl‐N‐isopropyltryptammonium fumarate according to any one of E50‐E52 as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone. [214] E57. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a therapeutically effective amount of 4‐methoxy‐ N‐methyl‐N‐isopropyltryptammonium fumarate according to E49. [215] E58. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline 4‐ methoxy‐N‐methyl‐N‐isopropyltryptammonium fumarate according to any one of E50‐E52. [216] E59. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a composition according to E53 or E55. [217] E60. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a composition according to E54 or E56. [218] E61. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a therapeutically effective amount of 4‐methoxy‐ N‐methyl‐N‐isopropyltryptammonium fumarate according to E49. [219] E62. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline 4‐ methoxy‐N‐methyl‐N‐isopropyltryptammonium fumarate according to any one of E50‐E52. [220] E63. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a composition according to E53 or E55. [221] E64. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a composition according to E54 or E56. [222] E65. Crystalline [2‐(4‐hydroxy‐1H‐indol‐3‐yl)ethyl](propan‐2‐yl)propylazanium (2E)‐3‐ carboxyprop‐2‐enoate (4‐hyroxy‐N‐propyl‐N‐isopropyltryptammnonium hydrofumarate).
[223] E66. Crystalline form 1 of [2‐(4‐hydroxy‐1H‐indol‐3‐yl)ethyl](propan‐2‐yl)propylazanium (2E)‐ 3‐carboxyprop‐2‐enoate (4‐hyroxy‐N‐propyl‐N‐isopropyltryptammnonium hydrofumarate). [224] E67. Crystalline form 1 of 4‐hyroxy‐N‐propyl‐N‐isopropyltryptammnonium hydrofumarate according to claim E66, characterized by at least one of: an orthorhombic crystal system at a temperature of about 297 K; a P2
12
12
1 space group at a temperature of about 297 K; unit cell dimensions a = 7.8854(7) Å, b = 12.7401(11) Å, c = 20.5577(18) Å, α = 90°, β = 90°, and ɣ = 90°; an X‐ray powder diffraction pattern substantially similar to FIG. 34; or an X‐ray powder diffraction pattern characterized by at least two peaks selected from 11.1, 12.0, and 15.8 °2θ ± 0.2 °2θ. [225] E68. A composition comprising crystalline 4‐hyroxy‐N‐propyl‐N‐isopropyltryptammnonium hydrofumarate according to any one of E65‐E67 and an excipient. [226] E69. A composition comprising crystalline 4‐hyroxy‐N‐propyl‐N‐isopropyltryptammnonium hydrofumarate according to any one of E65‐E67 as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone. [227] E70. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline 4‐ hyroxy‐N‐propyl‐N‐isopropyltryptammnonium hydrofumarate according to any one of E65‐E67. [228] E71. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a composition according to E68 or E69. [229] E72. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline 4‐ hyroxy‐N‐propyl‐N‐isopropyltryptammnonium hydrofumarate according to any one of E65‐E67. [230] E73. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a composition according to E68 or E69. [231] E74. 2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium 2‐{[2‐(5‐chloro‐1H‐indol‐3‐ yl)ethyl]azaniumyl}butanedioate (5‐chlorotryptammonium N‐succinyl‐5‐chlorotryptammonium). [232] E75. Crystalline 2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium 2‐{[2‐(5‐chloro‐1H‐indol‐3‐ yl)ethyl]azaniumyl}butanedioate (5‐chlorotryptammonium N‐succinyl‐5‐chlorotryptammonium).
[233] E76. Crystalline form 1 of 2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium 2‐{[2‐(5‐chloro‐1H‐ indol‐3‐yl)ethyl]azaniumyl}butanedioate (5‐chlorotryptammonium N‐succinyl‐5‐ chlorotryptammonium). [234] E77. Crystalline form 1 of 5‐chlorotryptammonium N‐succinyl‐5‐chlorotryptammonium according to E76, characterized by at least one of: a triclinic crystal system at a temperature of about 300 K;
a P1 ത space group at a temperature of about 300 K; unit cell dimensions a = 9.5806(13) Å, b = 11.1666(14) Å, c = 11.5518(17) Å, α = 87.333(5)°, β = 74.347(5)°, and ɣ = 85.637(5)°; an X‐ray powder diffraction pattern substantially similar to FIG. 35; or an X‐ray powder diffraction pattern characterized by at least two peaks selected from 12.8, 15.9, and 22.9 °2θ ± 0.2 °2θ. [235] E78. A composition comprising 5‐chlorotryptammonium N‐succinyl‐5‐chlorotryptammonium according to E74 and an excipient. [236] E79. A composition comprising crystalline 5‐chlorotryptammonium N‐succinyl‐5‐ chlorotryptammonium according to any one of E75‐E77 and an excipient. [237] E80. A composition comprising 5‐chlorotryptammonium N‐succinyl‐5‐chlorotryptammonium according to E74 as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone. [238] E81. A composition comprising crystalline 5‐chlorotryptammonium N‐succinyl‐5‐ chlorotryptammonium according to any one of E75‐E77 as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone. [239] E82. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a therapeutically effective amount of 5‐ chlorotryptammonium N‐succinyl‐5‐chlorotryptammonium according to E74. [240] E83. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline 5‐ chlorotryptammonium N‐succinyl‐5‐chlorotryptammonium according to any one of E75‐E77. [241] E84. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a composition according to E78 or E80.
[242] E85. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a composition according to E79 or E81. [243] E86. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a therapeutically effective amount of 5‐ chlorotryptammonium N‐succinyl‐5‐chlorotryptammonium according to E74. [244] E87. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline 5‐ chlorotryptammonium N‐succinyl‐5‐chlorotryptammonium according to any one of E75‐E77. [245] E88. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a composition according to E78 or E80. [246] E89. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a composition according to E79 or E81. [247] E90. 2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium 3‐carboxy‐2‐hydroxypropanoate (5‐ chlorotryptammonium hydromaleate). [248] E91. Crystalline 2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium 3‐carboxy‐2‐hydroxypropanoate (5‐chlorotryptammonium hydromaleate). [249] E92. Crystalline form 1 of 2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium 3‐carboxy‐2‐ hydroxypropanoate (5‐chlorotryptammonium hydromaleate). [250] E93. Crystalline form 1 of 5‐chlorotryptammonium hydromaleate according to E92, characterized by at least one of: a monoclinic crystal system at a temperature of about 300 K; a P2
1/c space group at a temperature of about 300 K; unit cell dimensions a = 9.6320(7) Å, b = 5.4224(4) Å, c = 28.494(2) Å, α = 90°, β = 95.758(3)°, and ɣ = 90°; an X‐ray powder diffraction pattern substantially similar to FIG. 36; or an X‐ray powder diffraction pattern characterized by at least two peaks selected from 9.2, 10.6, and 14.8 °2θ ± 0.2 °2θ. [251] E94. A composition comprising 5‐chlorotryptammonium hydromaleate according to E90 and an excipient. [252] E95. A composition comprising crystalline 5‐chlorotryptammonium hydromaleate according to any one of E91‐E93 and an excipient. [253] E96. A composition comprising 5‐chlorotryptammonium hydromaleate according to E90 as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic
drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone. [254] E97. A composition comprising crystalline 5‐chlorotryptammonium hydromaleate according to any one of E91‐E93 as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone. [255] E98. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a therapeutically effective amount of 5‐ chlorotryptammonium hydromaleate according to E90. [256] E99. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline 5‐ chlorotryptammonium hydromaleate according to any one of E91‐E93. [257] E100. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a composition according to E94 or E96. [258] E101. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a composition according to E95 or E97. [259] E102. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a therapeutically effective amount of 5‐ chlorotryptammonium hydromaleate according to E90. [260] E103. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline 5‐ chlorotryptammonium hydromaleate according to any one of E91‐E93. [261] E104. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a composition according to E94 or E96. [262] E105. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a composition according to E95 or E97. [263] E106. 2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium 2‐carboxybenzoate (5‐ chlorotryptammonium hydrophthalate). [264] E107. Crystalline 2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium 2‐carboxybenzoate (5‐ chlorotryptammonium hydrophthalate). [265] E108. Crystalline form 1 of 2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium 2‐carboxybenzoate (5‐chlorotryptammonium hydrophthalate).
[266] E109. Crystalline form 1 of 5‐chlorotryptammonium hydrophthalate according to E108, characterized by at least one of: a triclinic crystal system at a temperature of about 300 K;
a P1 ത space group at a temperature of about 300 K; unit cell dimensions a = 7.4013(6) Å, b = 9.8217(9) Å, c = 12.1614(12) Å, α = 83.285(3)°, β = 79.601(3)°, and ɣ = 78.437(3)°; an X‐ray powder diffraction pattern substantially similar to FIG. 37; or an X‐ray powder diffraction pattern characterized by at least two peaks selected from 11.3, 12.3, and 15.4 °2θ ± 0.2 °2θ. [267] E110. A composition comprising 5‐chlorotryptammonium hydrophthalate according to E106 and an excipient. [268] E111. A composition comprising crystalline 5‐chlorotryptammonium hydrophthalate according to any one of E107‐E109 and an excipient. [269] E112. A composition comprising 5‐chlorotryptammonium hydrophthalate according to E106 as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone. [270] E113. A composition comprising crystalline 5‐chlorotryptammonium hydrophthalate according to any one of E107‐E109 as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone. [271] E114. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a therapeutically effective amount of 5‐ chlorotryptammonium hydrophthalate according to E106. [272] E115. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline 5‐ chlorotryptammonium hydrophthalate according to any one of E107‐E109. [273] E116. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a composition according to E110 or E112. [274] E117. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a composition according to E111 or E113. [275] E118. A method of preventing or treating inflammation and/or pain comprising the step of:
administering to a subject in need thereof a therapeutically effective amount of 5‐ chlorotryptammonium hydrophthalate according to E106. [276] E119. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline 5‐ chlorotryptammonium hydrophthalate according to any one of E107‐E109. [277] E120. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a composition according to E110 or E112. [278] E121. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a composition according to E111 or E113. [279] E122. Bis(2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium) 2‐hydroxybutanedioate (5‐ chlorotryptammonium malate). [280] E123. Crystalline bis(2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium) 2‐hydroxybutanedioate (5‐ chlorotryptammonium malate). [281] E124. Crystalline form 1 of bis(2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium) 2‐ hydroxybutanedioate (5‐chlorotryptammonium malate). [282] E125. Crystalline form 1 of 5‐chlorotryptammonium malate according to E124, characterized by at least one of: a monoclinic crystal system at a temperature of about 300 K; a P2
1 space group at a temperature of about 300 K; unit cell dimensions a = 8.9975(8) Å, b = 12.3798(12) Å, c = 11.3628(11) Å, α = 90°, β = 106.617(3)°, and ɣ = 90°; an X‐ray powder diffraction pattern substantially similar to FIG. 38; or an X‐ray powder diffraction pattern characterized by at least two peaks selected from 12.5, 13.2, and 14.3 °2θ ± 0.2 °2θ. [283] E126. A composition comprising 5‐chlorotryptammonium malate according to E122 and an excipient. [284] E127. A composition comprising crystalline 5‐chlorotryptammonium malate according to any one of E123‐E125 and an excipient. [285] E128. A composition comprising 5‐chlorotryptammonium malate according to E122 as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone.
[286] E129. A composition comprising crystalline 5‐chlorotryptammonium malate according to any one of E123‐E125 as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone. [287] E130. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a therapeutically effective amount of 5‐ chlorotryptammonium malate according to E122. [288] E131. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline 5‐ chlorotryptammonium malate according to any one of E123‐E125. [289] E132. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a composition according to E126 or E128. [290] E133. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a composition according to E127 or E129. [291] E134. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a therapeutically effective amount of 5‐ chlorotryptammonium malate according to E122. [292] E135. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline 5‐ chlorotryptammonium malate according to any one of E123‐E125. [293] E136. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a composition according to E126 or E128. [294] E137. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a composition according to E127 or E129. [295] E138. 2‐(5‐methoxy‐1H‐indol‐3‐yl)ethan‐1‐aminium 2‐{[2‐(5‐methoxy‐1H‐indol‐3‐ yl)ethyl]azaniumyl}butanedioate (5‐methoxytryptammonium N‐succinyl‐5‐ methoxytryptammonium). [296] E139. Crystalline 2‐(5‐methoxy‐1H‐indol‐3‐yl)ethan‐1‐aminium 2‐{[2‐(5‐methoxy‐1H‐indol‐3‐ yl)ethyl]azaniumyl}butanedioate (5‐methoxytryptammonium N‐succinyl‐5‐ methoxytryptammonium). [297] E140. Crystalline form 1 of 2‐(5‐methoxy‐1H‐indol‐3‐yl)ethan‐1‐aminium 2‐{[2‐(5‐methoxy‐ 1H‐indol‐3‐yl)ethyl]azaniumyl}butanedioate (5‐methoxytryptammonium N‐succinyl‐5‐ methoxytryptammonium).
[298] E141. Crystalline form 1 of 5‐methoxytryptammonium N‐succinyl‐5‐ methoxytryptammonium according to E140, characterized by at least one of: a triclinic crystal system at a temperature of about 300 K;
a P1 ത space group at a temperature of about 300 K; unit cell dimensions a = 9.5838(10) Å, b = 10.9915(12) Å, c = 12.6894(11) Å, α = 87.708(3)°, β = 71.246(3)°, and ɣ = 84.805(4)°; an X‐ray powder diffraction pattern substantially similar to FIG. 39; or an X‐ray powder diffraction pattern characterized by at least two peaks selected from 7.4, 18.4, and 19.6 °2θ ± 0.2 °2θ. [299] E142. A composition comprising 5‐methoxytryptammonium N‐succinyl‐5‐ methoxytryptammonium according to E138 and an excipient. [300] E143. A composition comprising crystalline 5‐methoxytryptammonium N‐succinyl‐5‐ methoxytryptammonium according to any one of E139‐E141 and an excipient. [301] E144. A composition comprising 5‐methoxytryptammonium N‐succinyl‐5‐ methoxytryptammonium according to E138 as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone. [302] E145. A composition comprising crystalline 5‐methoxytryptammonium N‐succinyl‐5‐ methoxytryptammonium according to any one of E139‐E141 as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone. [303] E146. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a therapeutically effective amount of 5‐ methoxytryptammonium N‐succinyl‐5‐methoxytryptammonium according to E138. [304] E147. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline 5‐ methoxytryptammonium N‐succinyl‐5‐methoxytryptammonium according to any one of E139‐E141. [305] E148. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a composition according to E142 and E144. [306] E149. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a composition according to E143 and E145. [307] E150. A method of preventing or treating inflammation and/or pain comprising the step of:
administering to a subject in need thereof a therapeutically effective amount of 5‐ methoxytryptammonium N‐succinyl‐5‐methoxytryptammonium according to E138. [308] E151. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline 5‐ methoxytryptammonium N‐succinyl‐5‐methoxytryptammonium according to any one of E139‐E141. [309] E152. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a composition according to E142 or E144. [310] E153. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a composition according to E143 or E145. [311] E154. Crystalline 2‐(5‐methyl‐1H‐indol‐3‐yl)ethan‐1‐amine (5‐methyltryptamine). [312] E155. Crystalline form 1 of 2‐(5‐methyl‐1H‐indol‐3‐yl)ethan‐1‐amine (5‐methyltryptamine). [313] E156. Crystalline form 1 of 5‐methyltryptamine according to E155, characterized by at least one of: an orthorhombic crystal system at a temperature of about 300 K; a Pbca space group at a temperature of about 300 K; unit cell dimensions a = 9.1099(11) Å, b = 11.3907(11) Å, c = 19.154(2) Å, α = 90°, β = 90°, and ɣ = 90°; an X‐ray powder diffraction pattern substantially similar to FIG. 40; or an X‐ray powder diffraction pattern characterized by at least two peaks selected from 13.4, 15.5, and 19.5 °2θ ± 0.2 °2θ. [314] E157. A composition comprising crystalline 5‐methyltryptamine according to any one of 154‐ E156 and an excipient. [315] E158. A composition comprising crystalline 5‐methyltryptamine according to any one of E154‐E156 as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone. [316] E159. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline 5‐ methyltryptamine according to any one of E154‐E156. [317] E160. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a composition according to E157 or E158. [318] E161. A method of preventing or treating inflammation and/or pain comprising the step of:
administering to a subject in need thereof a therapeutically effective amount of crystalline 5‐ methyltryptamine according to any one of E154‐E156. [319] E162. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a composition according to E157 or E158. [320] E163. Crystalline bis(N‐[2‐(4‐hydroxy‐1H‐indol‐3‐yl)ethyl]‐N‐methylcyclopropanaminium) (2E)‐but‐2‐enedioate (4‐hydroxy‐N‐methyl‐N‐cyclopropyltryptammonium fumarate). [321] E164. Crystalline form 1 of bis(N‐[2‐(4‐hydroxy‐1H‐indol‐3‐yl)ethyl]‐N‐ methylcyclopropanaminium) (2E)‐but‐2‐enedioate (4‐hydroxy‐N‐methyl‐N‐ cyclopropyltryptammonium fumarate). [322] E165. Crystalline form 1 of 4‐hydroxy‐N‐methyl‐N‐cyclopropyltryptammonium fumarate according to E164, characterized by at least one of: a monoclinic crystal system at a temperature of about 300 K; a C2/c space group at a temperature of about 300 K; unit cell dimensions a = 19.5453(17) Å, b = 9.3265(7) Å, c = 17.4448(16) Å, α = 90°, β = 109.390(3)°, and ɣ = 90°; an X‐ray powder diffraction pattern substantially similar to FIG. 41; or an X‐ray powder diffraction pattern characterized by at least two peaks selected from 9.6, 10.7, and 16.6 °2θ ± 0.2 °2θ. [323] E166. A composition comprising crystalline 4‐hydroxy‐N‐methyl‐N‐ cyclopropyltryptammonium fumarate according to any one of E163‐E165 and an excipient. [324] E167. A composition comprising crystalline 4‐hydroxy‐N‐methyl‐N‐ cyclopropyltryptammonium fumarate according to any one of E163‐E165 as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone. [325] E168. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline 4‐ hydroxy‐N‐methyl‐N‐cyclopropyltryptammonium fumarate according to any one of E163‐E165. [326] E169. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a composition according to E166 or E167. [327] E170. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline 4‐ hydroxy‐N‐methyl‐N‐cyclopropyltryptammonium fumarate according to any one of E163‐E165.
[328] E171. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a composition according to E166 or E167. [329] E172. Crystalline [2‐(4‐hydroxy‐1H‐indol‐3‐yl)ethyl]bis(propan‐2‐yl)azanium (2E)‐3‐ carboxyprop‐2‐enoate (4‐hydroxy‐N,N‐diisopropyltryptammonium hydrofumarate). [330] E173. Crystalline form 1 of [2‐(4‐hydroxy‐1H‐indol‐3‐yl)ethyl]bis(propan‐2‐yl)azanium (2E)‐3‐ carboxyprop‐2‐enoate (4‐hydroxy‐N,N‐diisopropyltryptammonium hydrofumarate). [331] E174. Crystalline form 1 of 4‐hydroxy‐N,N‐diisopropyltryptammonium hydrofumarate according to E173, characterized by at least one of: an orthorhombic crystal system at a temperature of about 300 K; a P2
12
12
1space group at a temperature of about 300 K; unit cell dimensions a = 7.9541(3) Å, b = 12.5763(5) Å, c = 20.3351(6) Å, α = 90°, β = 90°, and ɣ = 90°; an X‐ray powder diffraction pattern substantially similar to FIG. 42; or an X‐ray powder diffraction pattern characterized by at least two peaks selected from 8.3, 11.2, and 15.8 °2θ ± 0.2 °2θ. [332] E175. A composition comprising crystalline 4‐hydroxy‐N,N‐diisopropyltryptammonium hydrofumarate according to any one of E172‐E174 and an excipient. [333] E176. A composition comprising crystalline 4‐hydroxy‐N,N‐diisopropyltryptammonium hydrofumarate according to any one of E172‐E174 as a first component and a second component selected from at least one of (a) a serotonergic drug, (b) a purified psilocybin derivative, (c) a purified cannabinoid, (d) a purified terpene, (e) an adrenergic drug, (f) a dopaminergic drug, (g) a monoamine oxidase inhibitor, (h) a purified erinacine, and (i) a purified hericenone. [334] E177. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline 4‐ hydroxy‐N,N‐diisopropyltryptammonium hydrofumarate according to any one of E172‐E174. [335] E178. A method of preventing or treating a psychological disorder comprising the step of: administering to a subject in need thereof a composition according to E175 or E176. [336] E179. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a therapeutically effective amount of crystalline 4‐ hydroxy‐N,N‐diisopropyltryptammonium hydrofumarate according to any one of E172‐E174. [337] E180. A method of preventing or treating inflammation and/or pain comprising the step of: administering to a subject in need thereof a composition according to E175 or E176. Examples [338] The preparation and characterization of each of crystalline form 1 of bis(2‐(5‐methoxy‐1H‐
indol‐3‐yl)ethan‐1‐aminium) (2E)‐but‐2‐enedioate (5‐methoxytryptammonium fumarate or 5‐MeO‐T fumarate), crystalline form 1 of 2‐(1H‐indol‐3‐yl)ethan‐1‐aminium 3‐carboxy‐2‐hydroxypropanoate (tryptammonium hydromalate), crystalline form 1 of 2‐(1H‐indol‐3‐yl)ethan‐1‐aminium 2‐ carboxybenzoate (tryptammonium hydrophthalate), crystalline form 1 of bis([2‐(4‐methoxy‐1H‐ indol‐3‐yl)ethyl](methyl)(propan‐2‐yl)azanium) (2E)‐but‐2‐enedioate (4‐methoxy‐N‐methyl‐N‐ isopropyltryptammonium fumarate or 4‐MeO‐MiPT fumarate), crystalline form 1 of [2‐(4‐hydroxy‐ 1H‐indol‐3‐yl)ethyl](propan‐2‐yl)propylazanium (2E)‐3‐carboxyprop‐2‐enoate (4‐hydroxy‐N‐propyl‐ N‐isopropyltryptammnonium hydrofumarate or 4‐HO‐PiPT hydrofumarate), crystalline form 1 of 2‐ (5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium 2‐{[2‐(5‐chloro‐1H‐indol‐3‐yl)ethyl]azaniumyl}butanedioate (5‐chlorotryptammonium N‐succinyl‐5‐chlorotryptammonium or 5‐Cl‐T∙N‐Suc‐5‐Cl‐T), crystalline form 1 of 2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium 3‐carboxy‐2‐hydroxypropanoate (5‐ chlorotryptammonium hydromaleate or 5‐Cl‐T hydromaleate), crystalline form 1 of 2‐(5‐chloro‐1H‐ indol‐3‐yl)ethan‐1‐aminium 2‐carboxybenzoate (5‐chlorotryptammonium hydrophthalate or 5‐Cl‐T hydrophthalate), crystalline form 1 of bis(2‐(5‐chloro‐1H‐indol‐3‐yl)ethan‐1‐aminium) 2‐ hydroxybutanedioate (5‐chlorotryptammonium malate or 5‐Cl‐T malate), crystalline form 1 of 2‐(5‐ methoxy‐1H‐indol‐3‐yl)ethan‐1‐aminium 2‐{[2‐(5‐methoxy‐1H‐indol‐3‐ yl)ethyl]azaniumyl}butanedioate (5‐methoxytryptammonium N‐succinyl‐5‐methoxytryptammonium or 5‐MeO‐T∙N‐Suc‐5‐MeO‐T), crystalline form 1 of 2‐(5‐methyl‐1H‐indol‐3‐yl)ethan‐1‐amine (5‐ methyltryptamine or 5‐Me‐T), crystalline form 1 of bis(N‐[2‐(4‐hydroxy‐1H‐indol‐3‐yl)ethyl]‐N‐ methylcyclopropanaminium) (2E)‐but‐2‐enedioate (4‐hydroxy‐N‐methyl‐N‐ cyclopropyltryptammonium fumarate or 4‐HO‐MCPT fumarate), and crystalline form 1 of [2‐(4‐ hydroxy‐1H‐indol‐3‐yl)ethyl]bis(propan‐2‐yl)azanium (2E)‐3‐carboxyprop‐2‐enoate (4‐hydroxy‐N,N‐ diisopropyltryptammonium hydrofumarate 4‐HO‐DiPT hydrofumarate) are described below. [339] Single Crystal X‐Ray Diffraction (SCXRD) Characterization: Data were collected on a Bruker D8 Venture CMOS Diffractometer equipped with an Oxford Cryosystems Cryostream cooling device and using Mo Kα radiation. Structures were solved using the Bruker SHELXTL program and refined with the SHELXTL program as part of the Bruker SHELXTL suite, or OLEX2 software. Unless otherwise stated, hydrogen atoms attached to carbon were placed geometrically and allowed to refine with a riding isotropic displacement parameter. Hydrogen atoms attached to a heteroatom were located in a difference Fourier synthesis and were allowed to refine freely with an isotropic displacement parameter. [340] Preparation and Characterization of Crystalline form 1 of 5‐MeO‐T fumarate
[341] Synthesis [342] 100 mg of 5‐methoxytryptamine freebase and 61 mg of fumaric acid were dissolved in 15 mL of methanol. The solution was refluxed under nitrogen for 12 hours and solvent was removed in vacuo. The residue was triturated with diethyl ether and filtered to yield the product as pale yellow powder. [343] Crystallization [344] Single crystals suitable for X‐ray diffraction studies were grown from the slow evaporation of an acetone/water solution. [345] Single Crystal Characterization [346] The single crystal data and structure refinement parameters for the crystalline form 1 structure of 5‐MeO‐T fumarate are reported in Table 3a, below. The data for crystalline form 1 of 5‐ MeO‐T fumarate in Table 3a relates to the asymmetric unit. [347] Preparation and Characterization of Crystalline form 1 of tryptammonium hydromalate [348] Synthesis [349] 100 mg of tryptamine and 125 mg of DL‐malic acid were dissolved in 10 mL of methanol. The solution was refluxed for 12 hours under an atmosphere of nitrogen. Solvent was removed in vacuo to yield a tan oil that was recrystallized from acetone. [350] Crystallization [351] Single crystals suitable for X‐ray diffraction studies were grown from the slow evaporation of an aqueous solution. [352] Single Crystal Characterization [353] The single crystal data and structure refinement parameters for the crystalline form 1 structure of tryptammonium hydromalate are reported in Table 3a, below. [354] Preparation and Characterization of Crystalline form 1 of tryptammonium hydrophthalate [355] Synthesis [356] 150 mg of tryptamine and 155 mg of phthalic acid were dissolved in 10 mL of methanol. The solution was refluxed for 12 hours under an atmosphere of nitrogen. Solvent was removed in vacuo to yield a brown oil that solidified to yield the hydrophthalate salt. [357] Crystallization [358] Single crystals suitable for X‐ray diffraction studies were grown from the slow evaporation of a methanol solution.
[359] Single Crystal Characterization [360] The single crystal data and structure refinement parameters for the crystalline form 1 structure of tryptammonium hydrophthalate are reported in Table 3a, below. [361] Preparation and Characterization of Crystalline form 1 of 4‐MeO‐MiPT fumarate [362] Synthesis [363] 100 mg of 4‐methoxy‐N‐methyl‐N‐isopropyltryptamine hydrofumarate and 104 mg of lead (II) acetate were dissolved in 15 mL of distilled water and allowed to stir for one hour. The resulting white precipitate (lead fumarate) was removed via filtration and the solvent of the filtrate was removed in vacuo. The resulting residue was picked up in acetone and filtered again. Solvent was removed in vacuo to yield a yellow oil which was recrystallized from acetone. [364] Crystallization [365] Single crystals suitable for X‐ray diffraction studies were grown from the slow evaporation of an acetone solution. [366] Single Crystal Characterization [367] The single crystal data and structure refinement parameters for the crystalline form 1 structure of 4‐MeO‐MiPT fumarate are reported in Table 3a, below. The data for crystalline form 1 of 4‐MeO‐MiPT fumarate in Table 3a relates to the asymmetric unit. [368] Preparation and Characterization of Crystalline form 1 of 4‐HO‐PiPT hydrofumarate [369] Crystallization [370] Single crystals suitable for X‐ray diffraction studies were grown from the slow evaporation of an isopropanol/acetone solution of a commercial sample (ChemLogix). [371] Single Crystal Characterization [372] The single crystal data and structure refinement parameters for the crystalline form 1 structure of 4‐HO‐PiPT hydrofumarate are reported in Table 3a, below. [373] Preparation and Characterization of Crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T [374] Synthesis [375] 76 mg of freebase 5‐chlorotryptamine and 23 mg of maleic acid were mixed together in methanol and heated at reflux for 48 hours. Solvent was removed in vacuo to yield solid product. [376] Crystallization [377] Single crystals suitable for X‐ray diffraction studies were grown from the slow evaporation of an acetone solution.
[378] Single Crystal Characterization [379] The single crystal data and structure refinement parameters for the crystalline form 1 structure of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T are reported in Table 3a, below. [380] Preparation and Characterization of Crystalline form 1 of 5‐Cl‐T hydromaleate [381] Synthesis [382] 75 mg of 5‐chlorotryptamine and 45 mg of maleic acid were dissolved in 15 mL of methanol. The solution was heated at reflux for 24 hours. Solvent was removed in vacuo to yield a solid which was recrystallized from methanol. [383] Crystallization [384] Single crystals suitable for X‐ray diffraction studies were grown from the slow evaporation of a methanol solution. [385] Single Crystal Characterization [386] The single crystal data and structure refinement parameters for the crystalline form 1 structure of 5‐Cl‐T hydromaleate are reported in Table 3a, below. [387] Preparation and Characterization of Crystalline form 1 of 5‐Cl‐T hydrophthalate [388] Synthesis [389] 82 mg of 5‐chlorotryptamine and 70 mg of phthalic acid were dissolved in 15 mL of methanol. The solution was heated at reflux for 48 hours. Solvent was removed in vacuo to yield the product as a powder. [390] Crystallization [391] Single crystals suitable for X‐ray diffraction studies were grown from the slow evaporation of a methanol solution. [392] Single Crystal Characterization [393] The single crystal data and structure refinement parameters for the crystalline form 1 structure of 5‐Cl‐T hydrophthalate are reported in Table 3b, below. [394] Preparation and Characterization of Crystalline form 1 of 5‐Cl‐T malate [395] Synthesis [396] 93 mg of 5‐chlorotryptamine and 32 mg of DL‐malic acid were dissolved in 20 mL of methanol. The solution was refluxed for 24 hours. Solvent was removed in vacuo to yield a crystalline solid. [397] Crystallization [398] Single crystals suitable for X‐ray diffraction studies were grown from the slow evaporation of a methanol solution.
[399] Single Crystal Characterization [400] The single crystal data and structure refinement parameters for the crystalline form 1 structure of 5‐Cl‐T malate are reported in Table 3b, below. [401] Preparation and Characterization of Crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T [402] Synthesis [403] 87 mg of 5‐methoxytryptamine freebase was mixed with 26 mg of maleic acid in methanol. The solution was heated at reflux for 48 hours. Solvent was removed in vacuo to yield a solid. [404] Crystallization [405] Single crystals suitable for X‐ray diffraction studies were grown from the slow evaporation of an acetone/water solution. [406] Single Crystal Characterization [407] The single crystal data and structure refinement parameters for the crystalline form 1 structure of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T are reported in Table 3b, below. [408] Preparation and Characterization of Crystalline form 1 of 5‐Me‐T [409] Synthesis [410] 506 mg of 5‐methyltryptamine hydrochloride was dissolved in 35 mL of dichloromethane. 193 mg of sodium hydroxide was dissolved in 35 mL of distilled water and this was added to the first solution. The biphasic mixture was stirred for two hours. The organic layer was isolated, washed four times, and dried over sodium sulfate. Solvent was removed in vacuo to yield the freebase. [411] Crystallization [412] Single crystals suitable for X‐ray diffraction studies were grown from the slow evaporation of a dichloromethane solution. [413] Single Crystal Characterization [414] The single crystal data and structure refinement parameters for the crystalline form 1 structure of 5‐Me‐T are reported in Table 3b, below. [415] Preparation and Characterization of Crystalline form 1 of 4‐HO‐MCPT fumarate [416] Crystallization [417] Crystals suitable for single crystal X‐ray diffraction studies were grown from the slow evaporation of a commercial sample (The Indole Shop). [418] Single Crystal Characterization [419] The single crystal data and structure refinement parameters for the crystalline form 1 structure of 4‐HO‐MCPT fumarate are reported in Table 3b, below. The data for crystalline form 1 of 4‐HO‐MCPT fumarate in Table 3b relates to the asymmetric unit.
[420] Preparation and Characterization of Crystalline form 1 of 4‐HO‐DiPT hydrofumarate [421] Crystallization [422] Crystals suitable for single crystal X‐ray analysis were grown from the slow evaporation of a methanol/water solution of a commercial sample from ChemLogix (Burnaby, BC Canada). [423] Single Crystal Characterization [424] The single crystal data and structure refinement parameters for the crystalline form 1 structure of 4‐HO‐DiPT hydrofumarate are reported in Table 3b, below. Table 3a





Table 3b




[425] FIG. 1 shows the molecular structure of crystalline form 1 of 5‐MeO‐T fumarate, showing the atomic labeling. [426] FIG. 2 shows the molecular structure of crystalline form 1 of tryptammonium hydromalate, showing the atomic labeling. [427] FIG. 3 shows the molecular structure of crystalline form 1 of tryptammonium hydrophthalate, showing the atomic labeling. [428] FIG. 4 shows the molecular structure of crystalline form 1 of 4‐MeO‐MiPT fumarate, showing the atomic labeling. [429] FIG. 5 shows the molecular structure of crystalline form 1 of 4‐HO‐PiPT hydrofumarate, showing the atomic labeling. [430] FIG. 6 shows the molecular structure of crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T, showing the atomic labeling. [431] FIG. 7 shows the molecular structure of crystalline form 1 of 5‐Cl‐T hydromaleate, showing the atomic labeling. [432] FIG. 8 shows the molecular structure of crystalline form 1 of 5‐Cl‐T hydrophthalate, showing the atomic labeling. [433] FIG. 9 shows the molecular structure of crystalline form 1 of 5‐Cl‐T malate, showing the atomic labeling. [434] FIG. 10 shows the molecular structure of crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T, showing the atomic labeling. [435] FIG. 11 shows the molecular structure of crystalline form 1 of 5‐Me‐T, showing the atomic labeling.
[436] FIG. 12 shows the molecular structure of crystalline form 1 of 4‐HO‐MCPT fumarate, showing the atomic labeling. [437] FIG. 13 shows the molecular structure of crystalline form 1 of 4‐HO‐DiPT hydrofumarate, showing the atomic labeling. [438] FIG. 14 shows the unit cell of crystalline form 1 of 5‐MeO‐T fumarate along the a‐axis. [439] FIG. 15 shows the unit cell of crystalline form 1 of tryptammonium hydromalate along the a‐ axis. [440] FIG. 16 shows the unit cell of crystalline form 1 of tryptammonium hydrophthalate along the b‐axis. [441] FIG. 17 shows the unit cell of crystalline form 1 of 4‐MeO‐MiPT fumarate along the c‐axis. [442] FIG. 18 shows the unit cell of crystalline form 1 of 4‐HO‐PiPT hydrofumarate along the a‐axis. [443] FIG. 19 shows the unit cell of crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T along the a‐axis. [444] FIG. 20 shows the unit cell of crystalline form 1 of 5‐Cl‐T hydromaleate along the b‐axis. [445] FIG. 21 shows the unit cell of crystalline form 1 of 5‐Cl‐T hydrophthalate along the c‐axis. [446] FIG. 22 shows the unit cell of crystalline form 1 of 5‐Cl‐T malate along the a‐axis. [447] FIG. 23 shows the unit cell of crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T along the a‐axis. [448] FIG. 24 shows the unit cell of crystalline form 1 of 5‐Me‐T along the a‐axis. [449] FIG. 25 shows the unit cell of crystalline form 1 of 4‐HO‐MCPT fumarate along the b‐axis. [450] FIG. 26 shows the unit cell of crystalline form 1 of 4‐HO‐DiPT hydrofumarate along the a‐ axis. [451] FIG. 27 shows the 2:1 ratio of crystalline form 1 of 5‐MeO‐T fumarate as a dimer. [452] FIG. 28 shows the 2:1 ratio of crystalline form 1 of 4‐MeO‐MiPT fumarate as a dimer. [453] FIG. 29 shows the 2:1 ratio of crystalline form 1 of 4‐HO‐MCPT fumarate as a dimer. [454] Simulated Powder X‐ray Diffraction (PXRD) Pattern [455] FIG. 30 shows a simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of 5‐MeO‐T fumarate generated from its single crystal data. Table 4 lists the angles, °2θ ± 0.2°2θ, and d‐spacing of the peaks identified in the experimental XRPD pattern of FIG. 30. The entire list of peaks, or a subset thereof, may be sufficient to characterize the cocrystal. For example, the cocrystal may be characterized by at least two peaks selected from the peaks at 7.3, 12.5, and 24.8 °2θ ± 0.2°2θ or their corresponding d‐spacing as well as by an XRPD pattern substantially similar to FIG. 30. [456] Simulated Powder X‐ray Diffraction (PXRD) Pattern [457] FIG. 31 shows a simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of tryptammonium hydromalate generated from its single crystal data. Table 5 lists the angles, °2θ ± 0.2°2θ, and d‐spacing of the peaks identified in the experimental XRPD pattern of FIG. 31. The entire
list of peaks, or a subset thereof, may be sufficient to characterize the cocrystal. For example, the cocrystal may be characterized by at least two peaks selected from the peaks at 7.1, 11.3, and 20.9 °2θ ± 0.2°2θ or their corresponding d‐spacing as well as by an XRPD pattern substantially similar to FIG. 31. [458] Simulated Powder X‐ray Diffraction (PXRD) Pattern [459] FIG. 32 shows a simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of tryptammonium hydrophthalate generated from its single crystal data. Table 6 lists the angles, °2θ ± 0.2°2θ, and d‐spacing of the peaks identified in the experimental XRPD pattern of FIG. 32. The entire list of peaks, or a subset thereof, may be sufficient to characterize the cocrystal. For example, the cocrystal may be characterized by at least two peaks selected from the peaks at 6.0, 11.2, and 15.4 °2θ ± 0.2°2θ or their corresponding d‐spacing as well as by an XRPD pattern substantially similar to FIG. 32. [460] Simulated Powder X‐ray Diffraction (PXRD) Pattern [461] FIG. 33 shows a simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of 4‐MeO‐MiPT fumarate generated from its single crystal data. Table 7 lists the angles, °2θ ± 0.2°2θ, and d‐spacing of the peaks identified in the experimental XRPD pattern of FIG. 33. The entire list of peaks, or a subset thereof, may be sufficient to characterize the cocrystal. For example, the cocrystal may be characterized by at least two peaks selected from the peaks at 9.1, 14.5, and 16.0 °2θ ± 0.2°2θ or their corresponding d‐spacing as well as by an XRPD pattern substantially similar to FIG. 33. [462] Simulated Powder X‐ray Diffraction (PXRD) Pattern [463] FIG. 34 shows a simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of 4‐HO‐PiPT hydrofumarate generated from its single crystal data. Table 8 lists the angles, °2θ ± 0.2°2θ, and d‐spacing of the peaks identified in the experimental XRPD pattern of FIG. 34. The entire list of peaks, or a subset thereof, may be sufficient to characterize the cocrystal. For example, the cocrystal may be characterized by at least two peaks selected from the peaks at 11.1, 12.0, and 15.8 °2θ ± 0.2°2θ or their corresponding d‐spacing as well as by an XRPD pattern substantially similar to FIG. 34. [464] Simulated Powder X‐ray Diffraction (PXRD) Pattern [465] FIG. 35 shows a simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T generated from its single crystal data. Table 9 lists the angles, °2θ ± 0.2°2θ, and d‐spacing of the peaks identified in the experimental XRPD pattern of FIG. 35. The entire list of peaks, or a subset thereof, may be sufficient to characterize the cocrystal. For example, the cocrystal may be characterized by at least two peaks selected from the peaks at 12.8, 15.9, and 22.9 °2θ ± 0.2°2θ or their corresponding d‐spacing as well as by an XRPD pattern substantially similar to FIG. 35.
[466] Simulated Powder X‐ray Diffraction (PXRD) Pattern [467] FIG. 36 shows a simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of 5‐Cl‐T hydromaleate generated from its single crystal data. Table 10 lists the angles, °2θ ± 0.2°2θ, and d‐spacing of the peaks identified in the experimental XRPD pattern of FIG. 36. The entire list of peaks, or a subset thereof, may be sufficient to characterize the cocrystal. For example, the cocrystal may be characterized by at least two peaks selected from the peaks at 9.2, 10.6, and 14.8 °2θ ± 0.2°2θ or their corresponding d‐spacing as well as by an XRPD pattern substantially similar to FIG. 36. [468] Simulated Powder X‐ray Diffraction (PXRD) Pattern [469] FIG. 37 shows a simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of 5‐Cl‐T hydrophthalate generated from its single crystal data. Table 11 lists the angles, °2θ ± 0.2°2θ, and d‐spacing of the peaks identified in the experimental XRPD pattern of FIG. 37. The entire list of peaks, or a subset thereof, may be sufficient to characterize the cocrystal. For example, the cocrystal may be characterized by at least two peaks selected from the peaks at 11.3, 12.3, and 15.4 °2θ ± 0.2°2θ or their corresponding d‐spacing as well as by an XRPD pattern substantially similar to FIG. 37. [470] Simulated Powder X‐ray Diffraction (PXRD) Pattern [471] FIG. 38 shows a simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of 5‐Cl‐T malate generated from its single crystal data. Table 12 lists the angles, °2θ ± 0.2°2θ, and d‐ spacing of the peaks identified in the experimental XRPD pattern of FIG. 38. The entire list of peaks, or a subset thereof, may be sufficient to characterize the cocrystal. For example, the cocrystal may be characterized by at least two peaks selected from the peaks at 12.5, 13.2, and 14.3 °2θ ± 0.2°2θ or their corresponding d‐spacing as well as by an XRPD pattern substantially similar to FIG. 38. [472] Simulated Powder X‐ray Diffraction (PXRD) Pattern [473] FIG. 39 shows a simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T generated from its single crystal data. Table 13 lists the angles, °2θ ± 0.2°2θ, and d‐spacing of the peaks identified in the experimental XRPD pattern of FIG. 39. The entire list of peaks, or a subset thereof, may be sufficient to characterize the cocrystal. For example, the cocrystal may be characterized by at least two peaks selected from the peaks at 7.4, 18.4, and 19.6 °2θ ± 0.2°2θ or their corresponding d‐spacing as well as by an XRPD pattern substantially similar to FIG. 39. [474] Simulated Powder X‐ray Diffraction (PXRD) Pattern [475] FIG. 40 shows a simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of 5‐Me‐T generated from its single crystal data. Table 14 lists the angles, °2θ ± 0.2°2θ, and d‐spacing of the peaks identified in the experimental XRPD pattern of FIG. 40. The entire list of peaks, or a subset thereof, may be sufficient to characterize the cocrystal. For example, the cocrystal may be
characterized by at least two peaks selected from the peaks at 13.4, 15.5, and 19.5 °2θ ± 0.2°2θ or their corresponding d‐spacing as well as by an XRPD pattern substantially similar to FIG. 40. [476] Simulated Powder X‐ray Diffraction (PXRD) Pattern [477] FIG. 41 shows a simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of 4‐HO‐MCPT fumarate generated from its single crystal data. Table 15 lists the angles, °2θ ± 0.2°2θ, and d‐spacing of the peaks identified in the experimental XRPD pattern of FIG. 41. The entire list of peaks, or a subset thereof, may be sufficient to characterize the cocrystal. For example, the cocrystal may be characterized by at least two peaks selected from the peaks at 9.6, 10.7, and 16.6 °2θ ± 0.2°2θ or their corresponding d‐spacing as well as by an XRPD pattern substantially similar to FIG. 41. [478] Simulated Powder X‐ray Diffraction (PXRD) Pattern [479] FIG. 42 shows a simulated X‐ray powder diffraction pattern (XRPD) for crystalline form 1 of 4‐HO‐DiPT hydrofumarate generated from its single crystal data. Table 16 lists the angles, °2θ ± 0.2°2θ, and d‐spacing of the peaks identified in the experimental XRPD pattern of FIG. 42. The entire list of peaks, or a subset thereof, may be sufficient to characterize the cocrystal. For example, the cocrystal may be characterized by at least two peaks selected from the peaks at 8.3, 11.2, and 15.8 °2θ ± 0.2°2θ or their corresponding d‐spacing as well as by an XRPD pattern substantially similar to FIG. 42. Table 4: Crystalline form 1 of 5‐MeO‐T fumarate


Table 5: Crystalline form 1 of tryptammonium hydromalate
Table 6: Crystalline form 1 of tryptammonium hydrophthalate
Table 7: Crystalline form 1 of 4‐MeO‐MiPT fumarate
Table 8: Crystalline form 1 of 4‐HO‐PiPT hydrofumarate
Table 9: Crystalline form 1 of 5‐Cl‐T∙N‐Suc‐5‐Cl‐T
Table 10: Crystalline form 1 of 5‐Cl‐T hydromaleate
Table 11: Crystalline form 1 of 5‐Cl‐T hydrophthalate
Table 12: Crystalline form 1 of 5‐Cl‐T malate
Table 13: Crystalline form 1 of 5‐MeO‐T∙N‐Suc‐5‐MeO‐T
Table 14: Crystalline form 1 of 5‐Me‐T
Table 15: Crystalline form 1 of 4‐HO‐MCPT fumarate
Table 16: Crystalline form 1 of 4‐HO‐DiPT hydrofumarate
References Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341. Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.