【0001】
【発明の属する技術分野】
本発明は、化粧料、洗浄剤、化学品等に有用な水酸基含有ポリマーの簡便で経済的な製法に関する。
【0002】
【従来の技術】
水酸基は代表的な親水性基であり、水酸基を有することで水溶性、水への濡れ性、金属酸化物への濡れ性、水への溶解性、水への分散性等を高いレベルで有するポリマーは多い。天然物由来の構造においては、水酸基を多く有するポリマーとして多糖類も利用されるが、多糖類には独特の物性があってあらゆる用途に適しているわけではなく、水酸基を有する合成ポリマーへの要望は強い。しかしながら、水酸基を有するポリマーの合成法は、煩雑である場合があり、特にポリオキシアルキレンを主鎖として、側鎖に水酸基を有する構造のポリマーを合成する方法は制限される。
【0003】
例えば、S.R.Sandlerらの論文(非特許文献1)、並びにE.J.Vandenbergの論文(非特許文献2)には、グリシドールのアニオン重合に関して述べられており、S.Penczekらの論文(非特許文献3)並びにR.Tokarらの論文(非特許文献4)にはグリシドールのカチオン重合に関して述べられているが、いずれも生成物は水酸基の級数変化やポリマー鎖の分岐が様々に含まれた複雑な構造の混合物である。エチレンオキシド等の他のアルキレンオキシドとグリシドールとの共重合も同様の機構で複雑な混合物となる。
【0004】
別の例としては、水酸基を保護したグリシドールの重合について、S.Tsurutaらの論文(非特許文献5)やE.J.Vandenbergの論文(非特許文献6)にはトリメチルシリルグリシジルエーテルやt−ブチルグリシジルエーテルの重合が述べられている。これらの重合は汎用なアルカリ金属水酸化物を開始剤としては進み難く、重合を円滑に進めるためには活性な有機金属化合物を開始剤あるいは触媒とする必要があり、その場合、高度な禁水・禁酸素といった反応環境の設定、及び反応後の開始剤や触媒の除去が煩雑である。また、トリメチルシリル基を有する化合物は高価で脱保護も煩雑であり、t−ブチルエーテル基の開裂には酢酸エステル化及び加水分解という2段階の反応を要して煩雑である。
【0005】
【非特許文献1】
J.Polym.Sci.Part A1,1966年,4巻,p.1253
【非特許文献2】
J.Polym.Sci.Poylm.Chem.,1985年,23巻,p.915
【非特許文献3】
J.Polym.Preprints,1992年,33巻,1号,p.156
【非特許文献4】
Macromolecules,1994年,27巻,p.320
【非特許文献5】
Macromol.Chem.,1968年,112巻,p.58
【非特許文献6】
J.Macromol.Sci.Chem.,1985年,A22巻,p.619
【0006】
【発明が解決しようとする課題】
本発明の課題は、従来の方法の問題点を解決し、簡便で経済的な水酸基含有ポリマーの製法を提供することにある。
【0007】
【課題を解決するための手段】
本発明者は、水酸基を有するモノマーを直接重合するのではなく、あるいは水酸基を高価な、又は脱保護に煩雑な構造で保護した状態で含有するモノマーを利用するのでもなく、アリルエーテル基を有するポリマーを異性化及び加水分解することにより、簡便で経済的に水酸基含有ポリマーを合成できることを見出した。
【0008】
即ち、本発明は、アリルエーテル基を有するポリマー(以下、ポリマーAという)に塩基触媒を作用させた後、得られた反応物を酸性加水分解する、水酸基含有ポリマーの製法を提供する。
【0009】
【発明の実施の形態】
[ポリマーA]
ポリマーAの構造は特に限定されないが、そのポリマーの主鎖骨格を構築した後にアリル化してアリルエーテル基を導入したものよりも、アリルエーテル基を有するモノマーを用いてアリルエーテル基を導入したものが好ましい。アリルエーテル基を有するモノマーとしては、アリルグリシジルエーテル等が挙げられる。
【0010】
ポリマーAの主鎖骨格は、ビニルモノマーをラジカル重合やイオン重合等で付加重合して得られるポリマー、ポリエーテル、ポリエステル、ポリアミド、ポリシロキサン等が好ましく、ポリエーテルが更に好ましく、ポリオキシアルキレンが特に好ましい。ポリオキシアルキレンは、オキシエチレン単位及び/又はオキシプロピレン単位からなるポリエーテル部分を有する構造が特に好ましい。これらポリマーが、多糖類やタンパク質類等に連結された構造として存在していても構わない。
【0011】
ポリマーAの合成法は特に限定されないが、主鎖がポリエーテルであるものに関しては塩基触媒の存在下で、アルキレンオキシドとアリルグリシジルエーテル等のアリルエーテル基を有するモノマーとを開環付加重合する反応が好ましい。この反応を用いれば、ポリマーAの合成に続いて温度を上げるか加熱時間を延ばすだけで、アリルエーテル基の異性化を行うことができるからである。この場合のアルキレンオキシド及びアリルエーテル基を有するモノマーの開環付加重合は、例えば、脂肪族アルコールを開始点として行えば脂肪族アルコールへのアルキレンオキシド及びアリルグリシジルエーテルの付加物という形でポリマーAが得られ、ヒドロキシエチルセルロースを開始点として行えば多糖鎖へのアルキレンオキシド及びアリルグリシジルエーテルの付加物という形でポリマーAが得られる等、特に限定されるものではない。
【0012】
ポリマーAの分子量について、基本的に本発明の合成方法の観点からの制約はないが、後述する測定方法において、数平均分子量が、500以上が好ましく、1000以上が更に好ましく、100万以下が好ましく、 50万以下が更に好ましい。また、直鎖状ポリマーでも、分岐したポリマーでも、架橋ゲル状のポリマーでも構わない。
【0013】
ポリマーAにおいて、ポリマーの分子量を、アリルエーテル基の平均数で割った値(以後、アリル基当量という)は、50以上が好ましく、100以上が更に好ましく、1万以下が好ましい。
【0014】
[水酸基含有ポリマーの製法]
ポリマーAに塩基触媒を作用させると、ポリマーA中のアリルエーテル基は、1−プロペニルエーテル基ヘ異性化すると考えられる。
【0015】
本発明に用いられる塩基触媒は、アルカリ金属水酸化物、アルカリ土類金属水酸化物、アルカリ金属アルコキシド、アルカリ土類金属アルコキシド、アルカリ金属アミド、あるいはアルカリ土類金属アミドが好ましく、これらの中でナトリウム、カリウム、セシウム、リチウム等のアルカリ金属の水酸化物が更に好ましい。
【0016】
塩基触媒はポリマーA100質量部に対して、0.1〜5 質量部用いることが好ましく、ポリマーAのアリルエーテル基1モルに対して、0.001〜0.1モル当量比用いることが好ましい。
【0017】
この異性化反応は、無溶媒で行っても、また塩基触媒を不活性化しないもので水と1級アルコール以外であれば溶媒を用いて行っても良い。ここで用いられる溶媒としては、エチレングリコールジメチルエーテル、エチレングリコールジエチルエーテル、ジエチレングリコールジメチルエーテル、ジエチレングリコールジエチルエーテル、トリエチレングリコールジメチルエーテル、トリエチレングリコールジエチルエーテル等のエーテル類が好ましい。溶媒を用いる場合、ポリマーA100質量部に対して、10〜200質量部用いることが好ましい。
【0018】
この異性化反応は、窒素やアルゴン等の不活性ガス雰囲気下で行われるのが好ましい。この異性化反応の温度は、温度が低すぎると異性化が極めて遅くなって実用性がなくなり、温度が高すぎるとポリマーの分解が起こることがあるので、70℃以上が好ましく、80℃以上180℃以下が更に好ましい。
【0019】
この異性化反応は、ポリマーAに含まれる全てのアリルエーテル基に対して必ずしも行われる必要はなく、最終的に用いる用途に対して適当な割合を異性化させれば良い。その異性化度合いの調節は、用いる塩基触媒の量や異性化温度や時間によって調節できる。本発明における異性化は、数分間のような瞬間的に完結するほど速いものではないので、異性化度合いを調節するのは容易である。
【0020】
ポリマーAに塩基触媒を作用させた後に得られる反応物の加水分解は、酸性水中で行われる。この際のpHは、低すぎると1−プロペニルエーテル基以外の部分が変化する可能性があり、また高すぎると加水分解が遅くなり実用に向かないので、1以上が好ましく、1.5以上が更に好ましく、5以下が好ましく、4以下が更に好ましい。
【0021】
酸性を得るための酸としては特に限定されないが、塩酸、硫酸、リン酸、硝酸等の無機酸、酢酸、シュウ酸、クエン酸、アクリル酸等の有機カルボン酸、メタンスルホン酸、トルエンスルホン酸等の硫黄含有有機酸等が例示される。なお、ヨウ化水素酸は1−プロペニルエーテル基以外に、特にエーテル結合の開列を起こす可能性が大きいので、好ましくない。
【0022】
加水分解は水溶液中で行われるが、反応溶媒として水が最も多く存在する必要はなく、酸性水中で化学反応を起こし難い有機溶媒であれば、共存しても良い。本反応に供するポリマーの溶解性によっては、有機溶剤の共存が必要であることもある。このような有機溶剤としては、炭素数1〜8の飽和あるいは不飽和の1価〜3価のアルコール、炭素数3〜5の脂肪族ケトン、あるいは前述のエーテル類が好ましく、これらを複数種併用しても構わない。加水分解時のポリマーの濃度は、溶液全体中で1〜90質量%が好ましく、5〜80質量%が更に好ましい。加水分解時の水の濃度は、溶液全体中で2〜99質量%が好ましく、10〜95質量%が更に好ましい。加水分解時の水以外の溶媒の濃度は、溶液全体中で40質量%以下が好ましく、30質量%以下が更に好ましい
加水分解の温度は、低すぎると、ポリマーが析出固化したり、水が固化して反応が極端に遅くなることがあり、高すぎると、水が無用に気化することで反応物内への存在が希薄になることがあり、加水分解が遅くなるので、10〜100℃が好ましく、20〜90℃が更に好ましい。加水分解の時間はpHと温度に依存するので、時間のみで限定されないが、通常は20分〜6時間の範囲で充分である。
【0023】
加水分解後は、異性化に要した塩基触媒とpH調節に使った酸との反応で生成する塩類、及びその当量関係よりも過剰の酸、必要に応じて使われた溶媒、加水分解で化学量論的に生成するプロピオンアルデヒド及びこの加水分解条件下で生成するプロピオンアルデヒドの誘導体等が、目的とする水酸基含有ポリマーと共に存在している。これらの除去方法は特に限定されず、減圧濃縮、凍結乾燥、共沸蒸留、再沈殿、液体による抽出、有機膜による透析、セラミック膜による限外ろ過、電気透析、活性炭等による吸着処理等、公知のあらゆる手法が、目的のポリマーを取り出すために適用できる。また用途によっては、そのような精製を特に施す必要がなく、加水分解後に生成する溶液をそのまま使用しても良い。
【0024】
本発明の製法に基づく反応式の一例を以下に示す。
【0025】
【化1】
【0026】
上記反応式中、nはエチレンオキシドの平均付加モル数を示す1以上の数であり、2〜500が好ましい。mはプロピレンオキシドの平均付加モル数を示す0以上の数であり、0〜100が好ましい。pはアリルグリシジルエーテルの平均付加モル数を示す1以上の数であり、2〜50が好ましい。qはアリルエーテル基が異性化した単位数を示す1以上の数であり、2〜50が好ましい。rはアリルエーテル基が異性化していない単位数を示す0以上の数であり、0〜48が好ましい。各構成単位の付加様式は限定されないが、アリルグリシジルエーテル単位はブロック状に並んでいないことが好ましい。
【0027】
本発明の製法により得られる水酸基含有ポリマーは、様々な親疎水性やイオン性や相溶性を持たせることができるので、分散剤、乳化剤、相溶化剤、皮膚や頭髪用感触改善剤、洗剤用ビルダー、シャンプーやリンス等のコンディショニング剤、コーティング剤、増粘剤、バインダー、繊維用柔軟剤、繊維用汚れ放出剤、繊維用仕上げ剤、繊維用滑剤等に好適なポリマーを本発明の方法によって製造できる。
【0028】
【実施例】
以下の例において、部及び%は特記しない限り、それぞれ質量部、質量%である。また、エチレンオキシドやアリルグリシジルエーテルの開環付加重合反応及び異性化反応は、ステンレススチール製のオートクレーブ中で行った。触媒に用いた水酸化カリウムは工業用グレードの板状ペレットであり、純度約96%(他は主に水分)である。また、加水分解反応はガラス製セパラブルフラスコ中窒素雰囲気下で行い、水にはイオン交換水を使用した。
【0029】
また、分子量及びアリル基の異性化率は以下の方法で測定した。
【0030】
<分子量>
ゲル浸透型液体クロマトグラフィー(GPC)によって行い、次の条件を用いた。溶離液及び添加塩類はいずれも液体クロマトグラフィー用のグレードの試薬から調製した。
・GPC条件
カラム:東ソー(株)製 α−M 2本、
溶離液:60mMリン酸、50mM臭化リチウムを含むジメチルホルムアミド、
検出器:示差屈折率計、
温度:40℃
標準:ポリエチレングリコール、ポリエチレンオキシド
測定濃度:5mg/ml
注入量:100μl。
【0031】
<アリル基の異性化率>
重水を溶媒としたプロトンNMRスペクトルにおける2重結合部分の変化率によって算出した。最終的に得られるポリマー中の、グリシド−ル単位の含有率は、その化合物にジメチルスルホキシドd6中で無水トリフルオロ酢酸を作用させ、グリシド−ル単位の水酸基をトリフルオロ酢酸エステルに変換することで他のシグナルと分離して観測・定量した。プロトンNMRスペクトルの測定には、Varian社製MERCURY400を用いた。
【0032】
合成例1:エチレンオキシド/アリルグリシジルエーテル共開環付加重合体▲1▼の合成
オートクレーブ内にトリエチレングリコール310部、及び水酸化カリウム9.26部を入れ、系内の空気を窒素に置換してから密封し、攪拌しながら加熱し、100℃に保った。このオートクレーブ内を1時間減圧(11Torr、すなわちおよそ1470Pa)にし、脱水を行った。その後窒素雰囲気下の0.02MPaに戻してから内温を120℃に上げた。
【0033】
ここに、エチレンオキシド2760部とアリルグリシジルエーテル690部との混合物(以後、エチレンオキシド混合物▲1▼という)を、反応槽内圧を0.4MPa以下に保ち、内温を125〜130℃に保てるように導入した。その際、エチレンオキシド混合物▲1▼全量の1/4が導入されるたびに一旦導入を留めて、同温度で30分間の熟成を行ってから続いて次なる導入を行った。エチレンオキシド混合物▲1▼の全量を導入後、同温度で90分間熟成して、エチレンオキシド/アリルグリシジルエーテル共開環付加重合体▲1▼(質量比 82:18、モル比92:8)を黄色糊状物として得た。数平均分子量は1450であった。
【0034】
実施例1:エチレンオキシド/アリルグリシジルエーテル/グリシドール共開環付加重合体の合成
合成例1で得た共開環付加重合体▲1▼を含む反応物を、窒素雰囲気下140℃に保って20時間攪拌した。共開環付加重合体▲1▼のアリルエーテル基の34%が1−プロペニルエーテル基に異性化したポリエーテルが黄色糊状物として得られた。
【0035】
この生成物150部にイオン交換水320部を加えて溶かし、攪拌しながら1N硫酸を加えてpHを2.38に調整してから、90〜91℃に1時間保った。生成した溶液を室温で攪拌し、ここに1N水酸化ナトリウムを加えてpHを7.02に調整し、透析用セルロースチューブ((株)日本メディカルサイエンスから販売されているヴィスキングチューブ)に詰めた。これを、緩やかに攪拌している30倍体積のイオン交換水に2日間浸し、イオン交換水を取り替えて再び同量のイオン交換水に2日間浸した。得られた淡黄色水溶液をセルロースチューブから取り出し、減圧乾燥して、エチレンオキシド/アリルグリシジルエーテル/グリシドール共開環付加重合体131部を黄色糊状物として得た。この生成物5%と無水トリフルオロ酢酸1%とを含むジメチルスルホキシドd6溶液を調製し、プロトンNMRスペクトルを観察したところ、得られたエチレンオキシド/アリルグリシジルエーテル/グリシドール共開環付加重合体におけるモノマー構成がこの順に92:5:3であることがわかった。
【0036】
実施例2:エチレンオキシド/グリシドール共開環付加重合体の合成
合成例1で得た共開環付加重合体▲1▼を含む反応物を、窒素雰囲気下140℃に保って77時間攪拌した。共開環付加重合体▲1▼のアリルエーテル基のほぼ全て(99%以上)が1−プロペニルエーテル基に異性化したポリエーテルが黄色糊状物として得られた。
【0037】
この生成物150部にイオン交換水320部を加えて溶かし、攪拌しながら1N硫酸を加えてpHを2.22に調整してから、90〜91℃に1時間保った。生成した溶液を室温で攪拌し、ここに1N水酸化ナトリウムを加えてpHを6.37に調整し、透析用セルロースチューブ((株)日本メディカルサイエンスから販売されているヴィスキングチューブ)に詰めた。これを、緩やかに攪拌している30倍体積のイオン交換水に2日間浸し、イオン交換水を取り替えて再び同量のイオン交換水に2日間浸した。得られた淡黄色水溶液をセルロースチューブから取り出し、減圧乾燥して、エチレンオキシド/グリシドール共開環付加重合体129部を黄色糊状物として得た。この生成物5%と無水トリフルオロ酢酸1%とを含むジメチルスルホキシドd6溶液を調製し、プロトンNMRスペクトルを観察したところ、得られたエチレンオキシド/グリシドール共開環付加重合体におけるモノマー構成がこの順に92:8であることがわかった。[0001]
TECHNICAL FIELD OF THE INVENTION
The present invention relates to a simple and economical method for producing a hydroxyl-containing polymer useful for cosmetics, detergents, chemicals, and the like.
[0002]
[Prior art]
The hydroxyl group is a representative hydrophilic group, and has a high level of water solubility, wettability to water, wettability to metal oxides, solubility in water, dispersibility in water, etc. by having a hydroxyl group. There are many polymers. In structures derived from natural products, polysaccharides are also used as polymers having a large number of hydroxyl groups, but polysaccharides have unique physical properties and are not suitable for all uses, and there is a demand for synthetic polymers having hydroxyl groups. Is strong. However, the method for synthesizing a polymer having a hydroxyl group may be complicated. In particular, a method for synthesizing a polymer having a polyoxyalkylene as a main chain and having a hydroxyl group in a side chain is limited.
[0003]
For example, R. Sandler et al. J. Vandenberg's article (Non-Patent Document 2) describes anionic polymerization of glycidol, Penczek et al. Tokar et al. (Non-Patent Document 4) describe the cationic polymerization of glycidol, but in each case, the product is a mixture of a complex structure containing various changes in the hydroxyl series and branching of the polymer chain. . Copolymerization of glycidol with other alkylene oxides such as ethylene oxide also results in complex mixtures by a similar mechanism.
[0004]
As another example, the polymerization of hydroxyl-protected glycidol has been described by S.H. Tsuruta et al. (Non-Patent Document 5) and E.I. J. Vandenberg's paper (Non-Patent Document 6) describes polymerization of trimethylsilyl glycidyl ether and t-butyl glycidyl ether. These polymerizations are difficult to proceed using a general-purpose alkali metal hydroxide as an initiator, and an active organometallic compound needs to be used as an initiator or a catalyst in order to smoothly carry out the polymerization. -Setting of the reaction environment such as oxygen-free and removal of the initiator and catalyst after the reaction are complicated. In addition, compounds having a trimethylsilyl group are expensive and complicated to deprotect, and cleavage of the t-butyl ether group requires two steps of acetic acid esterification and hydrolysis, which is complicated.
[0005]
[Non-patent document 1]
J. Polym. Sci. Part A1, 1966, vol. 4, p. 1253
[Non-patent document 2]
J. Polym. Sci. Poylm. Chem. 1985, Volume 23, p. 915
[Non-Patent Document 3]
J. Polym. Preprints, 1992, Vol. 33, No. 1, p. 156
[Non-patent document 4]
Macromolecules, 1994, Vol. 27, p. 320
[Non-Patent Document 5]
Macromol. Chem. , 1968, 112, p. 58
[Non-Patent Document 6]
J. Macromol. Sci. Chem. 1985, A22, p. 619
[0006]
[Problems to be solved by the invention]
An object of the present invention is to solve the problems of the conventional methods and to provide a simple and economical method for producing a hydroxyl group-containing polymer.
[0007]
[Means for Solving the Problems]
The present inventor does not directly polymerize a monomer having a hydroxyl group, or uses an monomer containing a hydroxyl group in a state protected by an expensive or complicated structure for deprotection, and has an allyl ether group. It has been found that a hydroxyl group-containing polymer can be easily and economically synthesized by isomerizing and hydrolyzing the polymer.
[0008]
That is, the present invention provides a method for producing a hydroxyl group-containing polymer, in which a base catalyst is allowed to act on a polymer having an allyl ether group (hereinafter, referred to as polymer A), and the obtained reaction product is subjected to acidic hydrolysis.
[0009]
BEST MODE FOR CARRYING OUT THE INVENTION
[Polymer A]
Although the structure of the polymer A is not particularly limited, a structure obtained by introducing an allyl ether group using a monomer having an allyl ether group is more preferable than a structure obtained by constructing a main chain skeleton of the polymer and then allylating and introducing an allyl ether group. preferable. Examples of the monomer having an allyl ether group include allyl glycidyl ether.
[0010]
The main chain skeleton of the polymer A is preferably a polymer obtained by addition polymerization of a vinyl monomer by radical polymerization or ionic polymerization, polyether, polyester, polyamide, polysiloxane, or the like, more preferably a polyether, and particularly preferably a polyoxyalkylene. preferable. As the polyoxyalkylene, a structure having a polyether moiety composed of oxyethylene units and / or oxypropylene units is particularly preferable. These polymers may exist as a structure linked to polysaccharides, proteins, and the like.
[0011]
The method for synthesizing the polymer A is not particularly limited, but for those having a main chain of polyether, a reaction of ring-opening addition polymerization of an alkylene oxide and a monomer having an allyl ether group such as allyl glycidyl ether in the presence of a base catalyst. Is preferred. If this reaction is used, the isomerization of the allyl ether group can be performed only by increasing the temperature or extending the heating time following the synthesis of the polymer A. In this case, the ring-opening addition polymerization of a monomer having an alkylene oxide and an allyl ether group is carried out, for example, when the aliphatic alcohol is used as a starting point, the polymer A is obtained as an adduct of the alkylene oxide and the allyl glycidyl ether to the aliphatic alcohol. It is not particularly limited, for example, if hydroxyethylcellulose is used as a starting point to obtain polymer A in the form of an adduct of alkylene oxide and allyl glycidyl ether to a polysaccharide chain.
[0012]
Although there is basically no restriction on the molecular weight of the polymer A from the viewpoint of the synthesis method of the present invention, in the measurement method described below, the number average molecular weight is preferably 500 or more, more preferably 1000 or more, and preferably 1,000,000 or less. And 500,000 or less is more preferable. Moreover, a linear polymer, a branched polymer, or a crosslinked gel polymer may be used.
[0013]
In the polymer A, the value obtained by dividing the molecular weight of the polymer by the average number of allyl ether groups (hereinafter referred to as allyl group equivalent) is preferably 50 or more, more preferably 100 or more, and preferably 10,000 or less.
[0014]
[Method for producing hydroxyl-containing polymer]
When a base catalyst acts on the polymer A, the allyl ether group in the polymer A is considered to be isomerized into a 1-propenyl ether group.
[0015]
The base catalyst used in the present invention is preferably an alkali metal hydroxide, an alkaline earth metal hydroxide, an alkali metal alkoxide, an alkaline earth metal alkoxide, an alkali metal amide, or an alkaline earth metal amide. Alkali metal hydroxides such as sodium, potassium, cesium and lithium are more preferred.
[0016]
The base catalyst is preferably used in an amount of 0.1 to 5 parts by mass with respect to 100 parts by mass of the polymer A, and is preferably used in a molar equivalent ratio of 0.001 to 0.1 with respect to 1 mol of the allyl ether group of the polymer A.
[0017]
This isomerization reaction may be carried out without a solvent, or may be carried out using a solvent that does not deactivate the base catalyst and is other than water and a primary alcohol. As the solvent used here, ethers such as ethylene glycol dimethyl ether, ethylene glycol diethyl ether, diethylene glycol dimethyl ether, diethylene glycol diethyl ether, triethylene glycol dimethyl ether, and triethylene glycol diethyl ether are preferable. When a solvent is used, it is preferable to use 10 to 200 parts by mass with respect to 100 parts by mass of the polymer A.
[0018]
This isomerization reaction is preferably performed in an atmosphere of an inert gas such as nitrogen or argon. If the temperature of the isomerization reaction is too low, the isomerization becomes extremely slow and practicability is lost, and if the temperature is too high, the polymer may be decomposed. C. or lower is more preferred.
[0019]
This isomerization reaction does not necessarily need to be performed for all the allyl ether groups contained in the polymer A, but may be performed at an appropriate ratio for the final use. The degree of isomerization can be adjusted by the amount of the base catalyst used, the isomerization temperature, and the time. Since the isomerization in the present invention is not so fast as to be completed instantaneously such as several minutes, it is easy to adjust the degree of isomerization.
[0020]
The hydrolysis of the reactant obtained after allowing the base catalyst to act on the polymer A is performed in acidic water. If the pH at this time is too low, the portion other than the 1-propenyl ether group may change, and if it is too high, the hydrolysis is slow and unsuitable for practical use. More preferably, it is 5 or less, more preferably 4 or less.
[0021]
The acid for obtaining the acidity is not particularly limited, but includes inorganic acids such as hydrochloric acid, sulfuric acid, phosphoric acid, and nitric acid, organic carboxylic acids such as acetic acid, oxalic acid, citric acid, and acrylic acid, methanesulfonic acid, and toluenesulfonic acid. And the like sulfur-containing organic acids. In addition, hydroiodic acid is not preferred because it is particularly likely to cause the opening of an ether bond other than the 1-propenyl ether group.
[0022]
Although the hydrolysis is performed in an aqueous solution, it is not necessary that water is the most present as a reaction solvent, and any organic solvent that does not easily cause a chemical reaction in acidic water may coexist. Depending on the solubility of the polymer used in this reaction, coexistence of an organic solvent may be necessary. As such an organic solvent, a saturated or unsaturated monohydric to trihydric alcohol having 1 to 8 carbon atoms, an aliphatic ketone having 3 to 5 carbon atoms, or the above-mentioned ethers are preferable. It does not matter. The concentration of the polymer during the hydrolysis is preferably from 1 to 90% by mass, more preferably from 5 to 80% by mass in the whole solution. The concentration of water at the time of hydrolysis is preferably 2 to 99% by mass, more preferably 10 to 95% by mass in the whole solution. The concentration of the solvent other than water during the hydrolysis is preferably 40% by mass or less in the whole solution, and more preferably 30% by mass or less. If the hydrolysis temperature is too low, the polymer is precipitated and solidified, or the water is solidified. The reaction may be extremely slow, and if it is too high, the presence in the reaction product may be diluted due to unnecessary vaporization of water, and the hydrolysis may be delayed. Preferably, 20 to 90 ° C is more preferable. The hydrolysis time depends on pH and temperature, and is not limited only by the time, but usually in the range of 20 minutes to 6 hours is sufficient.
[0023]
After hydrolysis, the salts formed by the reaction of the base catalyst required for the isomerization with the acid used for pH adjustment, the acid in excess of its equivalence, the solvent used as necessary, Stoichiometrically formed propionaldehyde and a derivative of propionaldehyde formed under the hydrolysis conditions are present together with the desired hydroxyl group-containing polymer. These removal methods are not particularly limited, and known methods such as vacuum concentration, freeze-drying, azeotropic distillation, reprecipitation, extraction with a liquid, dialysis with an organic membrane, ultrafiltration with a ceramic membrane, electrodialysis, adsorption treatment with activated carbon, etc. Can be applied to remove the desired polymer. In some applications, such purification is not particularly required, and a solution formed after hydrolysis may be used as it is.
[0024]
An example of a reaction formula based on the production method of the present invention is shown below.
[0025]
Embedded image
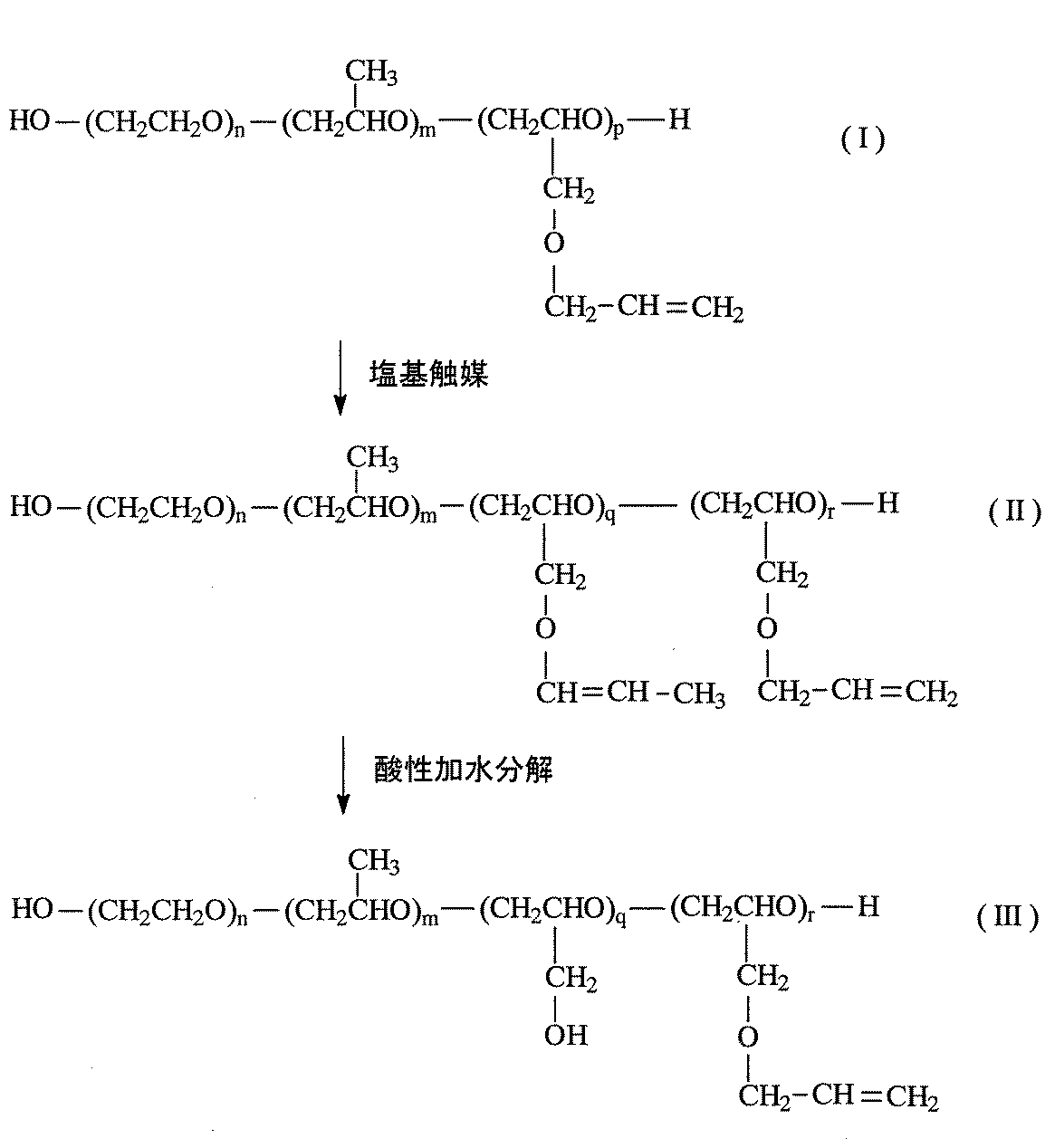
In the above reaction formula, n is a number of 1 or more indicating an average addition mole number of ethylene oxide, and preferably 2 to 500. m is a number of 0 or more indicating the average number of moles of propylene oxide added, and preferably 0 to 100. p is a number of 1 or more indicating the average addition mole number of allyl glycidyl ether, and preferably 2 to 50. q is a number of 1 or more indicating the number of units in which the allyl ether group is isomerized, and preferably 2 to 50. r is a number of 0 or more indicating the number of units in which the allyl ether group is not isomerized, and preferably 0 to 48. The mode of addition of each structural unit is not limited, but the allyl glycidyl ether units are preferably not arranged in a block.
[0027]
Since the hydroxyl group-containing polymer obtained by the production method of the present invention can have various hydrophilic / hydrophobic properties, ionicity and compatibility, dispersants, emulsifiers, compatibilizers, feel improvers for skin and hair, detergent builders Polymers suitable for conditioning agents such as shampoos and rinses, coating agents, thickeners, binders, softeners for fibers, stain release agents for fibers, finishing agents for fibers, lubricants for fibers, etc. can be produced by the method of the present invention. .
[0028]
【Example】
In the following examples, parts and% are parts by mass and mass%, respectively, unless otherwise specified. The ring-opening addition polymerization reaction and isomerization reaction of ethylene oxide and allyl glycidyl ether were performed in a stainless steel autoclave. The potassium hydroxide used for the catalyst is an industrial-grade plate-like pellet having a purity of about 96% (others are mainly water). The hydrolysis reaction was performed in a glass separable flask under a nitrogen atmosphere, and ion-exchanged water was used as water.
[0029]
The molecular weight and the isomerization ratio of the allyl group were measured by the following methods.
[0030]
<Molecular weight>
It was performed by gel permeation type liquid chromatography (GPC), and the following conditions were used. Both eluents and added salts were prepared from grade reagents for liquid chromatography.
-GPC condition column: two α-M manufactured by Tosoh Corporation,
Eluent: dimethylformamide containing 60 mM phosphoric acid, 50 mM lithium bromide,
Detector: Differential refractometer,
Temperature: 40 ° C
Standard: polyethylene glycol, polyethylene oxide Measurement concentration: 5 mg / ml
Injection volume: 100 μl.
[0031]
<Isomerization ratio of allyl group>
It was calculated from the rate of change of the double bond in a proton NMR spectrum using heavy water as a solvent. In finally obtained polymer, glycidol - content of Le units thereof by the action of trifluoroacetic anhydride to the compound in dimethyl sulfoxide d 6, glycidol - converting the Le units hydroxyl groups trifluoroacetate Was observed and quantified separately from other signals. For the measurement of the proton NMR spectrum, MERCURY400 manufactured by Varian was used.
[0032]
Synthesis Example 1: Synthesis of ethylene oxide / allyl glycidyl ether co-ring-opening addition polymer (1) 310 parts of triethylene glycol and 9.26 parts of potassium hydroxide were placed in an autoclave, and air in the system was replaced with nitrogen. And heated to 100 ° C. with stirring. The inside of the autoclave was depressurized (11 Torr, that is, about 1470 Pa) for 1 hour to perform dehydration. Then, the internal temperature was raised to 120 ° C. after returning to 0.02 MPa in a nitrogen atmosphere.
[0033]
Here, a mixture of 2760 parts of ethylene oxide and 690 parts of allyl glycidyl ether (hereinafter referred to as ethylene oxide mixture (1)) was introduced such that the internal pressure of the reactor was maintained at 0.4 MPa or less and the internal temperature was maintained at 125 to 130 ° C. did. At that time, each time 1/4 of the total amount of the ethylene oxide mixture (1) was introduced, the introduction was stopped once, aging was performed at the same temperature for 30 minutes, and then the next introduction was performed. After the entire amount of the ethylene oxide mixture (1) was introduced, the mixture was aged at the same temperature for 90 minutes to give an ethylene oxide / allyl glycidyl ether co-ring-opening addition polymer (1) (mass ratio 82:18, molar ratio 92: 8) as a yellow paste. Obtained as a solid. The number average molecular weight was 1450.
[0034]
Example 1: Synthesis of ethylene oxide / allyl glycidyl ether / glycidol co-ring-opening addition polymer A reaction product containing co-ring-opening addition polymer (1) obtained in Synthesis Example 1 was kept at 140 ° C in a nitrogen atmosphere for 20 hours. Stirred. Polyether in which 34% of the allyl ether groups of the co-ring-opening addition polymer (1) were isomerized into 1-propenyl ether groups was obtained as a yellow paste.
[0035]
To 150 parts of this product, 320 parts of ion-exchanged water was added to dissolve, and the pH was adjusted to 2.38 by adding 1N sulfuric acid with stirring, and then maintained at 90 to 91 ° C for 1 hour. The resulting solution was stirred at room temperature, adjusted to pH 7.02 by adding 1N sodium hydroxide, and packed in a dialysis cellulose tube (Visking tube sold by Nippon Medical Science Co., Ltd.). . This was immersed in gently stirred 30-fold volume ion-exchanged water for 2 days, replaced with ion-exchanged water, and immersed again in the same amount of ion-exchanged water for 2 days. The obtained pale yellow aqueous solution was taken out of the cellulose tube and dried under reduced pressure to obtain 131 parts of ethylene oxide / allyl glycidyl ether / glycidol co-ring-opening addition polymer as a yellow paste. Dimethylsulfoxide d 6 solution containing the product 5% and 1% of trifluoroacetic anhydride was prepared and observation of the proton NMR spectrum, the monomer in the resulting ethylene / allyl glycidyl ether / glycidol co ring opening addition polymers The structure was found to be 92: 5: 3 in this order.
[0036]
Example 2: Synthesis of ethylene oxide / glycidol co-ring-opening addition polymer A reaction product containing the co-ring-opening addition polymer (1) obtained in Synthesis Example 1 was stirred at 140 ° C under a nitrogen atmosphere for 77 hours. Almost all (99% or more) of the allyl ether groups of the co-ring-opening addition polymer (1) were isomerized into 1-propenyl ether groups to obtain a polyether as a yellow paste.
[0037]
To 150 parts of the product, 320 parts of ion-exchanged water was added to dissolve, and 1N sulfuric acid was added with stirring to adjust the pH to 2.22, and then kept at 90 to 91 ° C for 1 hour. The resulting solution was stirred at room temperature, 1N sodium hydroxide was added to adjust the pH to 6.37, and the solution was packed in a dialysis cellulose tube (Visking tube sold by Nippon Medical Science Co., Ltd.). . This was immersed in gently stirred 30-fold volume ion-exchanged water for 2 days, replaced with ion-exchanged water, and immersed again in the same amount of ion-exchanged water for 2 days. The obtained pale yellow aqueous solution was taken out of the cellulose tube and dried under reduced pressure to obtain 129 parts of an ethylene oxide / glycidol co-ring-opening addition polymer as a yellow paste. Dimethylsulfoxide d 6 solution containing the product 5% and 1% of trifluoroacetic anhydride was prepared and observation of the proton NMR spectrum, the monomers constituting the resulting ethylene / glycidol co ring opening addition polymers in this order 92: 8.