Disclosure of Invention
The invention aims to provide a preparation method of (2R,3S,4S) -4-amino-2-tetradecyl pyrrolidine-3-alcohol.
The invention also aims to provide the application of the (2R,3S,4S) -4-amino-2-tetradecyl pyrrolidine-3-ol in preparing anti-cancer drugs.
The invention is realized by the following technical scheme:
a preparation method of (2R,3S,4S) -4-amino-2-tetradecyl pyrrolidine-3-ol (P1) is provided, wherein the structural formula of the (2R,3S,4S) -4-amino-2-tetradecyl pyrrolidine-3-ol (P1) is shown in the specification
Comprises the following steps:
the preparation method of the (2R,3S,4S) -4-amino-2-tetradecylpyrrolidin-3-ol (P1) comprises the following steps:
s1: (3R,4R,5R) -5-allyl-4- ((4-methoxybenzyl) oxy) -1- ((4-nitrophenyl) sulfonyl) pyrrolidin-3-ol (compound 1), reacted with trifluoromethanesulfonic anhydride, and then treated with benzylamine to give 4-amino substituted compound (3S,4S,5R) -5-allyl-N-benzyl-4- ((4-methoxybenzyl) oxy) -1- ((2-nitrophenyl) sulfonyl) pyrrolidin-3-amine (compound 2);
s2: after the (3S,4S,5R) -5-allyl-N-benzyl-4- ((4-methoxybenzyl) oxy) -1- ((2-nitrophenyl) sulfonyl) pyrrolidine-3-amine (compound 2) and 1-tridecene are subjected to olefin metathesis reaction, o-nitrobenzenesulfonyl is removed, hydrogen double bond reduction is carried out, benzyl is removed, and then P-methoxybenzyl is removed, so that (2R,3S,4S) -4-amino-2-tetradecyl pyrrolidine-3-ol (compound P1) is obtained.
In the preparation method of the (2R,3S,4S) -4-amino-2-tetradecyl pyrrolidine-3-ol, the reaction temperature of the benzylamine treatment in the step S1 is 30-40 ℃, and the reaction time is 0.5-1.5 h. Preferably, the reaction temperature of the benzylamine treatment in the step S1 is 35 ℃, and the reaction time is 1 h.
In the above preparation method of (2R,3S,4S) -4-amino-2-tetradecylpyrrolidin-3-ol, S1 comprises the following steps:
dissolving (3R,4R,5R) -5-allyl-4- ((4-methoxybenzyl) oxy) -1- ((4-nitrophenyl) sulfonyl) pyrrolidine-3-ol (compound 1) in dichloromethane, adding pyridine, dropwise adding trifluoromethanesulfonic anhydride at-20 ℃ under the protection of nitrogen, reacting for 20min at-20 ℃, washing an organic phase with hydrochloric acid (1M), a saturated sodium bicarbonate solution, ice water and saturated common salt water, drying anhydrous magnesium sulfate, filtering, evaporating the solvent under reduced pressure, directly dissolving the obtained crude product in benzylamine, reacting for 0.5-1.5h at 30-40 ℃ under the protection of nitrogen, and directly separating and purifying the reaction solution by using a silica gel column chromatography to obtain (3S,4S,5R) -5-allyl-N-benzyl-4- ((4-methoxybenzyl) oxy) -1- ((2-nitrophenyl) sulfonyl) pyrrolidin-3-amine (Compound 2).
Preferably, the S1 includes the following steps: dissolving (3R,4R,5R) -5-allyl-4- ((4-methoxybenzyl) oxy) -1- ((4-nitrophenyl) sulfonyl) pyrrolidine-3-ol (compound 1) in dichloromethane, adding pyridine, dropwise adding trifluoromethanesulfonic anhydride at-20 ℃ under the protection of nitrogen, reacting for 20min at-20 ℃, washing an organic phase with hydrochloric acid (1M), a saturated sodium bicarbonate solution, ice water and saturated salt water, drying anhydrous magnesium sulfate, filtering, evaporating the solvent under reduced pressure, directly dissolving the obtained crude product in benzylamine, reacting for 1h at 35 ℃ under the protection of nitrogen, and directly separating and purifying the reaction liquid by using a silica gel column chromatography to obtain (3S,4S,5R) -5-allyl-N-benzyl-4- ((4-methoxybenzyl) oxy) -1- ((2-nitre) Phenylphenyl) sulfonyl) pyrrolidin-3-amine (compound 2).
In the above preparation method of (2R,3S,4S) -4-amino-2-tetradecylpyrrolidin-3-ol, ceric ammonium nitrate is used as a reactant in the step of removing p-methoxybenzyl in step S2.
In the above preparation method of (2R,3S,4S) -4-amino-2-tetradecylpyrrolidin-3-ol, S2 comprises the following steps: dissolving (3S,4S,5R) -5-allyl-N-benzyl-4- ((4-methoxybenzyl) oxy) -1- ((2-nitrophenyl) sulfonyl) pyrrolidin-3-amine (Compound 2) in dichloromethane, and adding 1-tridecene, Grubbs under nitrogen2ndHeating and refluxing the catalyst for 7 hours to obtain a reaction solutionCooling to room temperature, filtering through diatomite, eluting with ethyl acetate, and rotary evaporating to remove solvent to obtain crude olefin compound; dissolving the crude olefin compound in acetonitrile, adding cesium carbonate and thiophenol under the protection of nitrogen, reacting for 3h at 50 ℃, cooling the reaction liquid to room temperature, filtering through diatomite, eluting with dichloromethane, and removing the solvent by rotary evaporation to obtain the crude compound without o-nitrobenzenesulfonyl; the crude compound obtained is dissolved in methanol and 20% Pd (OH) is added2Reacting 30 wt% of/C with 10 wt% of Pd/C (10 wt%) for 24 hours in a hydrogen atmosphere, filtering the reaction liquid by using kieselguhr, and spin-drying the solvent to obtain a crude product of the reduction product without benzyl; dissolving the benzyl-removed crude product of the reduction product in acetonitrile/water (1:4), adding ammonium ceric nitrate, reacting for 3h at room temperature, and directly separating and purifying the reaction liquid by using a silica gel column chromatography to obtain (2R,3S,4S) -4-amino-2-tetradecyl pyrrolidine-3-alcohol (compound P1).
In the present invention, the temperature of the hydrochloric acid, the saturated sodium bicarbonate solution, and the saturated brine is 0 ℃.
The concentration of the hydrochloric acid is 1M.
The ratio of acetonitrile/water (1:4) is volume ratio.
The 20% Pd (OH)2The amount of the/C is 30 wt%, and the amount of the 10% Pd/C is 10 wt%.
Application of (2R,3S,4S) -4-amino-2-tetradecyl pyrrolidine-3-alcohol (P1) in preparing anticancer drugs. The cancer includes lung cancer, colon cancer, melanoma, and liver cancer.
The application of (2R,3S,4S) -4-amino-2-tetradecyl pyrrolidine-3-alcohol in preparing a medicament for treating lung cancer.
Application of (2R,3S,4S) -4-amino-2-tetradecyl pyrrolidine-3-alcohol in preparing medicine for treating colon cancer.
Application of (2R,3S,4S) -4-amino-2-tetradecyl pyrrolidine-3-alcohol in preparing medicine for treating melanoma.
The application of (2R,3S,4S) -4-amino-2-tetradecyl pyrrolidine-3-alcohol in preparing medicine for treating liver cancer.
Application of (2R,3S,4S) -4-amino-2-tetradecyl pyrrolidine-3-alcohol in preparing medicines for inhibiting human lung cancer A549 cell proliferation.
Application of (2R,3S,4S) -4-amino-2-tetradecyl pyrrolidine-3-alcohol in preparation of medicines for inhibiting human colon cancer LOVO cell proliferation.
The application of (2R,3S,4S) -4-amino-2-tetradecyl pyrrolidine-3-alcohol in preparing a medicament for inhibiting the cell proliferation of a human melanoma cell A375.
The application of (2R,3S,4S) -4-amino-2-tetradecyl pyrrolidine-3-alcohol in preparing a medicament for inhibiting the cell proliferation of human liver cancer HepG 2.
The invention has the beneficial effects that:
(1) the preparation method provided by the invention has the characteristics of no need of intermediate separation, mild reaction conditions, gram-scale achievement, simple operation, high reproducibility and the like.
(2) In the synthesis of intermediate compound 2, the reaction conditions of compound 1 and trifluoromethanesulfonic anhydride are-20 ℃ to 0 ℃ as reported in the literature, and the subsequent bimolecular nucleophilic substitution reaction takes benzylamine as solvent and takes reaction at 50 ℃ for 40 hours, so that the yield of 2 steps is only 50%, and side products are generated (24%). The inventor finds that the reaction condition of the compound 1 and the trifluoromethanesulfonic anhydride is-20 ℃, the subsequent bimolecular nucleophilic substitution reaction only needs to be heated to 35 ℃, the raw material point completely disappears in 1 hour, no by-product is generated due to overhigh temperature, the reaction yield is 78%, and the reaction yield is improved by nearly 30% compared with that reported in the literature.
(3) The reaction for synthesizing the target compound P1 from the intermediate 2 is a separate 4-step reaction reported in the literature, wherein 3 steps need to be separated and purified by silica gel column chromatography, and the operation is complex. In addition, p-methoxybenzyl (PMB) was difficult to remove, the hydrogen reduction time required 4 days or more, the reaction time was long, the yield was 44%, and the reproducibility was low, and the average yield of 4 steps was 10% when the experiment was repeated. Although the method for synthesizing P1 from 2 is completed through 4 steps, only the final product P1 needs to be purified, when the P-methoxybenzyl (PMB) protecting group is removed, the reaction is shortened to 3 hours by using Ceric Ammonium Nitrate (CAN), the operation is simple, the total yield of 4 steps is 41%, and the repeatability is high.
(4) The result of the experiment on the proliferation activity of the anti-tumor cells shows that (2R,3S,4S)) IC of 4-amino-2-tetradecylpyrrolidin-3-ol on human Lung cancer cells (A549), human Colon cancer cells (LOVO), human melanoma cells (A375), human hepatoma cells (HepG2)50The values are all less than 10 mu M, and the synthetic drug IC meets the requirements specified in the preclinical research guiding principle of new traditional Chinese medicine50The inhibitor is considered to have certain inhibition effect when the concentration is less than or equal to 10 mu M. The compound (2R,3S,4S) -4-amino-2-tetradecyl pyrrolidine-3-alcohol has certain inhibiting effect on human lung cancer cells (A549), human colon cancer cells (LOVO), human melanoma cells (A375) and human liver cancer cells (HepG2), wherein the inhibiting effect on IC of the human lung adenocarcinoma cells (A549), the human colon cancer cells (LOVO) and the human liver cancer cells (HepG2)50Are all lower than 0.1 mu M, which shows that the compound (2R,3S,4S) -4-amino-2-tetradecyl pyrrolidine-3-alcohol has strong inhibition effect on the proliferation of human lung adenocarcinoma (A549), human colon cancer (LOVO) and human liver cancer (HepG 2).
Detailed Description
The present invention will be further described with reference to specific embodiments so that those skilled in the art may better understand the present invention, but the present invention is not limited thereto.
EXAMPLE 1 Synthesis of intermediate Compound 2
Compound 1(1.08g,2.4mmol) was dissolved in dichloromethane (9.6mL), pyridine (396 μ L,4.8mmol) was added, trifluoromethanesulfonic anhydride (605 μ L,3.6mmol) was added dropwise at-20 ℃ under nitrogen, the reaction was carried out for 20min, and the disappearance of the starting material spot was detected by TLC (ethyl acetate-petroleum ether ═ 1: 2). The reaction solution was diluted with dichloromethane, washed with 0 ℃ 1M hydrochloric acid, 0 ℃ saturated sodium bicarbonate, ice water, 0 ℃ saturated common salt in this order, dried over anhydrous magnesium sulfate, filtered, and the solvent was removed under reduced pressure, the crude product was directly dissolved in benzylamine (7.2mL), reacted at 35 ℃ for 1 hour under nitrogen protection, and the reaction solution was separated and purified by silica gel column chromatography (ethyl acetate-dichloromethane-petroleum ether ═ 3:3:4) to obtain compound 2(0.787g, 61%).1H NMR(CDCl3,600MHz)δ(ppm):7.94(dd,J=8.1,1.2Hz,1H),7.56–7.61(m,1H),7.48– 7.54(m,2H),7.19–7.32(m,5H),7.00(d,J=8.6Hz,2H),6.77–6.83(m,2H),5.73–5.82(m, 1H),5.05–5.15(m,2H),4.33(d,J=11.2Hz,1H),4.09–4.16(m,2H),3.80(s,3H),3.72(dd,J= 9.1,7.1Hz,1H),3.63–3.68(m,2H),3.60(d,J=13.1Hz,1H),3.40(ddd,J=9.7,7.1,4.0Hz,1H), 3.12(t,J=9.3Hz,1H),2.56–2.63(m,1H),2.14–2.22(m,1H),1.85(s,1H).13C NMR(CDCl3, 150MHz)δ(ppm):159.39,148.72,139.72,133.56,133.27,131.15,130.64,130.39,129.46, 129.28,128.49,128.05,127.24,123.79,118.70,113.76,78.42,70.42,63.38,57.61,55.32,51.96, 51.62,38.86.HRMS(ESI):m/zcalcd for C28H32N3O6S(M+H)+:538.2012.found:538.2009.
Example 2 Synthesis of intermediate Compound 2
Compound 1(0.36g,0.8mmol) was dissolved in dichloromethane (5.0mL), pyridine (132 μ L,1.6mmol) was added, trifluoromethanesulfonic anhydride (202 μ L,1.2mmol) was added dropwise at-20 ℃ under nitrogen, the reaction was carried out for 20min, and the disappearance of the starting material spot was detected by TLC (ethyl acetate-petroleum ether ═ 1: 2). The reaction solution was diluted with dichloromethane, washed with 1M hydrochloric acid at 0 ℃, saturated sodium bicarbonate at 0 ℃, ice water, and saturated common salt at 0 ℃, dried over anhydrous magnesium sulfate, filtered, and the solvent was removed under reduced pressure, the crude product was directly dissolved in benzylamine (3.0mL), reacted at 35 ℃ for 1 hour under nitrogen protection, and the reaction solution was separated and purified by silica gel column chromatography (ethyl acetate-dichloromethane-petroleum ether ═ 3:3:4) to obtain compound 2(0.335g, 78%).
EXAMPLE 3 Synthesis of intermediate Compound 2
Compound 1(1.44g,3.2mmol) was dissolved in dichloromethane (20.0ml), pyridine (528 μ L,6.4mmol) was added, trifluoromethanesulfonic anhydride (808 μ L,4.8mmol) was added dropwise at-20 ℃ under nitrogen, the reaction was carried out for 20min, and the disappearance of the starting material spot was detected by TLC (ethyl acetate-petroleum ether ═ 1: 2). The reaction solution was diluted with dichloromethane, washed with 0 ℃ 1M hydrochloric acid, 0 ℃ saturated sodium bicarbonate, ice water, 0 ℃ saturated common salt in this order, dried over anhydrous magnesium sulfate, filtered, and the solvent was removed under reduced pressure, the crude product was directly dissolved in benzylamine (12.0mL), reacted at 35 ℃ for 1 hour under nitrogen protection, and the reaction solution was separated and purified by silica gel column chromatography (ethyl acetate-dichloromethane-petroleum ether ═ 3:3:4) to give compound 2(1.308g, 76%).
EXAMPLE 4 preparation of (2R,3S,4S) -4-amino-2-tetradecylpyrrolidin-3-ol (P1)
Compound 2(100mg,0.186mmol) was dissolved in dichloromethane (6mL) and 1-tridecene (136mg,0.744mmol), Grubbs, added under nitrogen
2nd(31mg,0.037mmol) and the reaction was heated to reflux for 7h and the starting material disappeared by TLC (3: 2:5 ethyl acetate-dichloromethane-petroleum ether). The reaction was cooled to room temperature and filtered through celite, eluting with ethyl acetate to give a brown crude. The crude product was dissolved in acetonitrile (6mL), and cesium carbonate (121mg,0.372mmol), thiophenol (31mg,0.28mmol) were added under nitrogen protection, reacted at 50 ℃ for 3h, and the disappearance of the starting material was detected by TLC (methanol-dichloromethane ═ 7: 93). The reaction was cooled to room temperature and filtered through celite, eluting with dichloromethane, to give a brown crude. Dissolving the obtained crude product in methanol (6mL), adding 20% palladium on carbon (30mg) and 10% palladium on carbon (10 mg), reacting for 24h under hydrogen atmosphere, filtering the reaction solution with diatomite, and spin-drying the solvent to obtain colorless oily crude product. The resulting crude product was dissolved in acetonitrile/water (1:4), and cerium ammonium nitrate (174mg,0.372mmol) was added to react at room temperature for 3 hours. The reaction mixture was directly subjected to silica gel column chromatography (methanol-ethanol-dichloromethane-aqueous ammonia: 6:12:77:5) to give (2R,3S,4S) -4-amino-2-tetradecylpyrrolidin-3-ol (23mg, 41%) as a white solid.
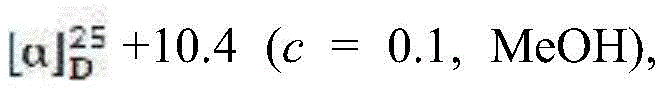
H NMR (CDCl
3,400MHz)δ(ppm):3.48(t,J=5.6Hz,1H),3.29–3.43(m,2H),2.82(q,J=5.6Hz, 1H),2.60(dq,J=11.6,6.0,5.5Hz,1H),1.84(brs,4H),1.54–1.63(m,1H),1.25(s,25H),0.88(t, J=6.7Hz,3H).
13C NMR(CDCl
3,100MHz)δ(ppm):75.72,65.00,53.15,51.73,34.02,31.85, 29.55,29.58(2C),29.62(4C),29.48,29.29,26.75,22.61,14.00.IR(KBr,cm
-1):3348,3242,2918, 2850,1558,1469,1458,1377,1350,1118,1080,1028,956,908,894,869,825,719.HRMS(ESI): m/zcalcd for C
18H
38N
2O(M+H)
+:299.3062.found:299.3047.
Example 5 preparation of (2R,3S,4S) -4-amino-2-tetradecylpyrrolidin-3-ol (P1)
Compound 2(50mg,0.093mmol) was dissolved in dichloromethaneTo an alkane (5mL), 1-tridecene (68mg,0.372mmol), Grubbs, was added under nitrogen2nd(16mg,0.019mmol) and the reaction was heated under reflux for 7h and the starting material disappeared by TLC (ethyl acetate-dichloromethane-petroleum ether: 3:2: 5). The reaction was cooled to room temperature and filtered through celite, eluting with ethyl acetate to give a brown crude. The crude product was dissolved in acetonitrile (5mL), and cesium carbonate (60 mg,0.186mmol), thiophenol (16mg,0.14mmol) were added under nitrogen protection, reacted at 50 ℃ for 3h, and the disappearance of the starting material was detected by TLC (methanol-dichloromethane ═ 7: 93). The reaction was cooled to room temperature and filtered through celite, eluting with dichloromethane, to give a brown crude. Dissolving the obtained crude product in methanol (5mL), adding 20% palladium on carbon (15mg) and 10% palladium on carbon (5mg), reacting for 24h under hydrogen atmosphere, filtering the reaction solution with diatomite, and spin-drying the solvent to obtain colorless oily crude product. The crude product was dissolved in acetonitrile/water (1:4), and cerium ammonium nitrate (87mg,0.186mmol) was added to react at room temperature for 3 hours. The reaction mixture was directly subjected to silica gel column chromatography (methanol-ethanol-dichloromethane-aqueous ammonia: 6:12:77:5) to give (2R,3S,4S) -4-amino-2-tetradecylpyrrolidin-3-ol (10mg, 36%) as a white solid.
Example 6 preparation of (2R,3S,4S) -4-amino-2-tetradecylpyrrolidin-3-ol (P1)
Compound 2(150mg,0.279mmol) was dissolved in dichloromethane (15mL) and 1-tridecene (144mg,1.116mmol), Grubbs, added under nitrogen2nd(48mg,0.057mmol) and the reaction was heated to reflux for 7h and the starting material disappeared by TLC (3: 2:5 ethyl acetate-dichloromethane-petroleum ether). The reaction was cooled to room temperature and filtered through celite, eluting with ethyl acetate to give a brown crude. The crude product was dissolved in acetonitrile (15mL), and cesium carbonate (180mg,0.558mmol), thiophenol (48mg,0.42mmol) were added under nitrogen, reacted at 50 ℃ for 3h, and the disappearance of the starting material was detected by TLC (methanol-dichloromethane ═ 7: 93). The reaction was cooled to room temperature and filtered through celite, eluting with dichloromethane, to give a brown crude. Dissolving the obtained crude product in methanol (15mL), adding 20% palladium on carbon (45mg) and 10% palladium on carbon (15mg), reacting for 24h under hydrogen atmosphere, filtering the reaction solution with diatomite, and spin-drying the solvent to obtain colorless oily crude product. The crude product obtained is dissolved in acetonitrile/water (1:4),cerium ammonium nitrate (261mg,0.558mmol) was added thereto, and the mixture was reacted at room temperature for 3 hours. The reaction mixture was directly subjected to silica gel column chromatography (methanol-ethanol-dichloromethane-aqueous ammonia: 6:12:77:5) to give (2R,3S,4S) -4-amino-2-tetradecylpyrrolidin-3-ol as a white solid (28mg, 34%).
The pharmacological tests and results of the compounds of the invention are as follows:
experimental example 1: research on antitumor activity of compound of the invention
1.1 Experimental materials
Compound P1 was dissolved in dimethyl sulfoxide (DMSO, final concentration 0.4%) and prepared to 1mg/mL in 15% fetal bovine serum RPMI-1640 medium, and diluted to the desired concentration. Human lung adenocarcinoma cells (A549), human colon carcinoma cells (LOVO), human melanoma cells (A375), human hepatoma cells (HepG2) and human esophageal carcinoma cells (TE-1), all purchased from cell banks of Chinese academy of sciences, cultured in DMEM (high glucose) medium containing 15% fetal bovine serum, placed at 37 ℃ in 5% CO2Culturing in an incubator.
1.2 antitumor Activity test
1.2.1 MTT preparation method
Preparation of 5mg/mL MTT solution: weighing 500.0mg of MTT powder, dissolving in warm 100mL PBS, filtering with 0.22 μm pore size microporous membrane to obtain filtrate, subpackaging in autoclaved EP tube, and freezing at-20 deg.C in dark place.
1.2.2 cell culture and Experimental methods
Taking out the cryopreservation tube with the tumor cells from the liquid nitrogen, quickly putting the tube into a37 ℃ incubator, and shaking continuously until the tube is melted. After wiping the edge of the cryopreserving tube cover with 75% alcohol, sucking the cell suspension, transferring the cell suspension into a 10mL centrifuge tube, and supplementing 5mL culture medium. Centrifuging at low speed (25 deg.C, 3000r/min,5min), discarding supernatant, adding culture medium, and centrifuging once again. Diluting with appropriate amount of culture medium, blowing off cells with a straw to obtain suspension, transferring into a culture flask, and standing at 37 deg.C and 5% CO2Culturing in a cell culture box. The culture medium is replaced the next day, and the mixture is placed at 37 ℃ and 5% CO2And continuing culturing in the cell culture box.
Human lung adenocarcinoma cell (A549), human noduleIntestinal cancer cells (LOVO), human melanoma cells (A375), human liver cancer cells (HepG2) and human esophageal cancer cells (TE-1) are adherent cells, adherent tumor cells in logarithmic growth phase are washed according to the growth rate of the tumor cells, and the number of cells is adjusted to 1 × 10 by digesting with 0.25% EDTA pancreatin5Perml/mL in 96-well plates, 100. mu.L per well, at 37 ℃ in CO2Culturing in incubator, and administering after 24 h. The administration groups were dosed with different concentrations of drug (compound P1), each drug being provided in 5 dose groups of 100.00, 10.00, 1.00, 0.10, 0.01 μ M each, with three duplicate wells per concentration. A blank control, DMSO (0.8%) solvent control, and a cisplatin positive control were set. CO at 37 deg.C2After culturing in an incubator for 48 hours, the OD value was measured by the MTT method, and the cell inhibition ratio was calculated.
1.2.3 cellular IC50Calculation of values
After culturing human lung adenocarcinoma cells (A549), human colon cancer cells (LOVO), human melanoma cells (A375), human liver cancer cells (HepG2) and human esophageal cancer cells (TE-1) for 48h, the culture was terminated, and then 10. mu.L of 0.5% MTT solution was added to each well and placed in CO2In the incubator, after 4 hours, the liquid in each well was removed, then 0.2mL of DMSO solution was added, followed by sufficiently shaking with low frequency on a shaker to sufficiently dissolve formazan crystallized in blue-violet, placing in an enzyme-labeling instrument, recording OD at 490nm, calculating the average OD of three parallel wells of different concentrations, and calculating the cell inhibition ratio and IC of each test drug at different concentrations based on the average50Values (see table 1).
The inhibition rate (%) [ 1-test product OD value/negative control group OD value ] × 100%.
1.2.4 determination of results
The guiding principle of preclinical research of new Chinese medicine shows that the natural product IC50The inhibitor is considered to have certain inhibiting effect when the concentration is less than or equal to 30 mu M; synthesis of drug IC50The inhibitor is considered to have certain inhibition effect when the concentration is less than or equal to 10 mu M.
TABLE 1 IC of (2R,3S,4S) -4-amino-2-tetradecylpyrrolidin-3-ol against tumor cell proliferation50Value of
1.3 results and discussion
The result of the anti-tumor cell proliferation activity experiment shows that the IC of (2R,3S,4S) -4-amino-2-tetradecyl pyrrolidine-3-alcohol in human lung adenocarcinoma cells (A549), human colon carcinoma cells (LOVO), human melanoma cells (A375) and human liver cancer cells (HepG2)50The values are all less than 10 mu M, and the synthetic drug IC meets the requirements specified in the preclinical research guiding principle of new traditional Chinese medicine50The inhibitor is considered to have certain inhibition effect when the concentration is less than or equal to 10 mu M. The compound (2R,3S,4S) -4-amino-2-tetradecyl pyrrolidine-3-alcohol has certain inhibiting effect on human lung adenocarcinoma cells (A549), human colon cancer cells (LOVO), human melanoma cells (A375) and human liver cancer cells (HepG2), wherein the inhibiting effect on IC of the human lung adenocarcinoma cells (A549), the human colon cancer cells (LOVO) and the human liver cancer cells (HepG2)50All are lower than 0.1 mu M, which shows that the compound (2R,3S,4S) -4-amino-2-tetradecyl pyrrolidine-3-alcohol has strong inhibition effect on human lung adenocarcinoma (A549), human colon cancer (LOVO) and human liver cancer (HepG2) cells.